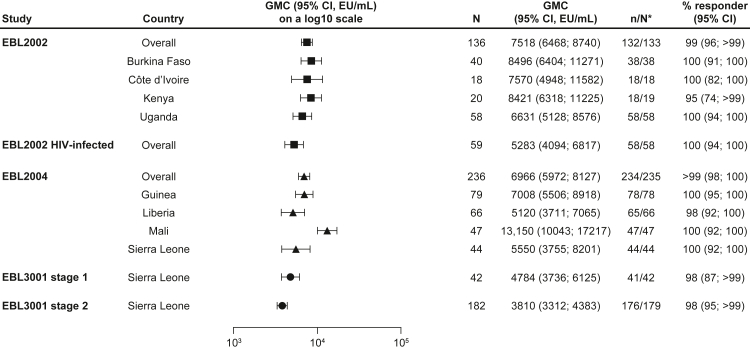
Fig. 2.
EBOV GP-binding antibody GMCs in adult participants at 21 or 28 days post-dose 2, stratified by country. EBOV GP-binding antibody GMCs at 21 or 28 days post-dose 2 are presented overall for adults who received the Ad26.ZEBOV, MVA-BN-Filo vaccine regimen in EBL2002 (n = 136), HIV-infected adults in EBL2002 (n = 59), adults in EBL2004 (n = 236), and adults in EBL 3001 stage 1 (n = 42) and stage 2 (n = 182), as well as by country in each study. Samples were analysed according to Q2 Solution's FANG ELISA standard operating procedure, and a single reportable value for each participant sample at each timepoint was uploaded for statistical analysis. Error bars represent 95% CIs. CI; Confidence interval. EBOV GP; Ebola virus glycoprotein. ELISA; Enzyme-linked immunosorbent assay. EU; ELISA unit. FANG; Filovirus Animal Nonclinical Group. GMC; Geometric mean concentration. ∗N is the number of participants with data at baseline and at 21 days (EBL2002/EBL3001) or 28 days (EBL2004) post-dose 2.