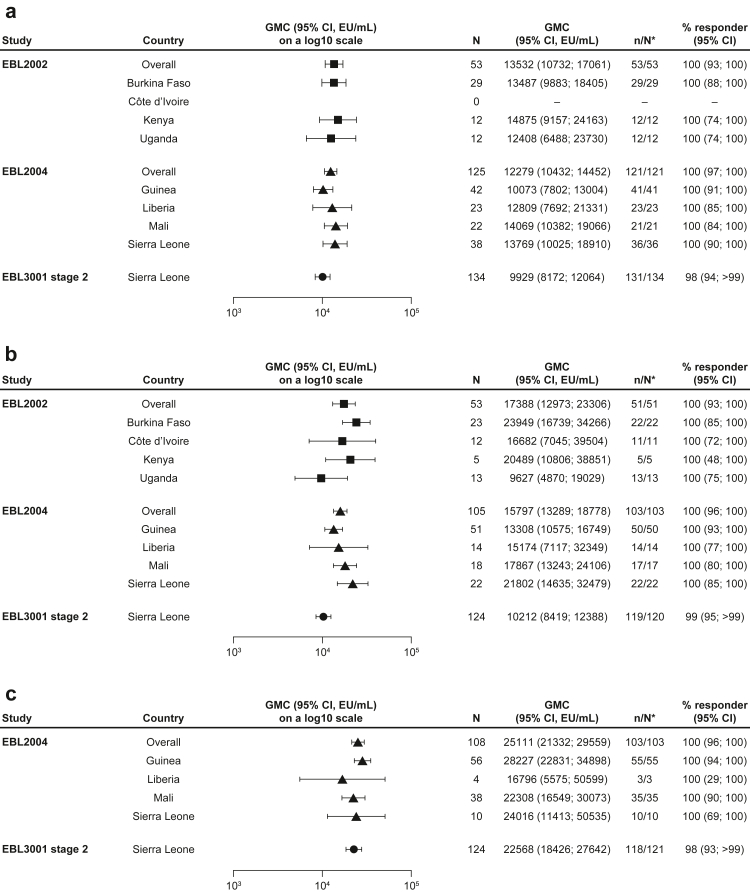
Fig. 3.
EBOV-GP binding antibody GMCs in (a) adolescents, (b) older children, and (c) younger children at 21 or 28 days post-dose 2, stratified by country. EBOV GP-binding antibody GMCs at 21 or 28 days post-dose 2 are presented overall for paediatric participants who received the Ad26.ZEBOV, MVA-BN-Filo vaccine regimen, including adolescents aged 12–17 years in EBL2002 (n = 53), EBL2004 (n = 125), and EBL3001 stage 2 (n = 134); older children aged 4–11 years in EBL2002 (n = 53) and EBL3001 stage 2 (n = 124) and 5–11 years in EBL2004 (n = 105); and younger children aged 1–4 years EBL2004 (n = 108) and 1–3 years in EBL3001 stage 2 (n = 124). GMCs are also shown by country for each study. Samples were analysed according to Q2 Solution's FANG ELISA standard operating procedure, and a single reportable value for each participant sample at each timepoint was uploaded for statistical analysis. Error bars represent 95% CIs. CI; Confidence interval. EBOV GP; Ebola virus glycoprotein. ELISA; Enzyme-linked immunosorbent assay. EU; ELISA unit. FANG; Filovirus Animal Nonclinical Group. GMC; Geometric mean concentration. ∗N is the number of participants with data at baseline and at 21 days (EBL2002/EBL3001) or 28 days (EBL2004) post-dose 2.