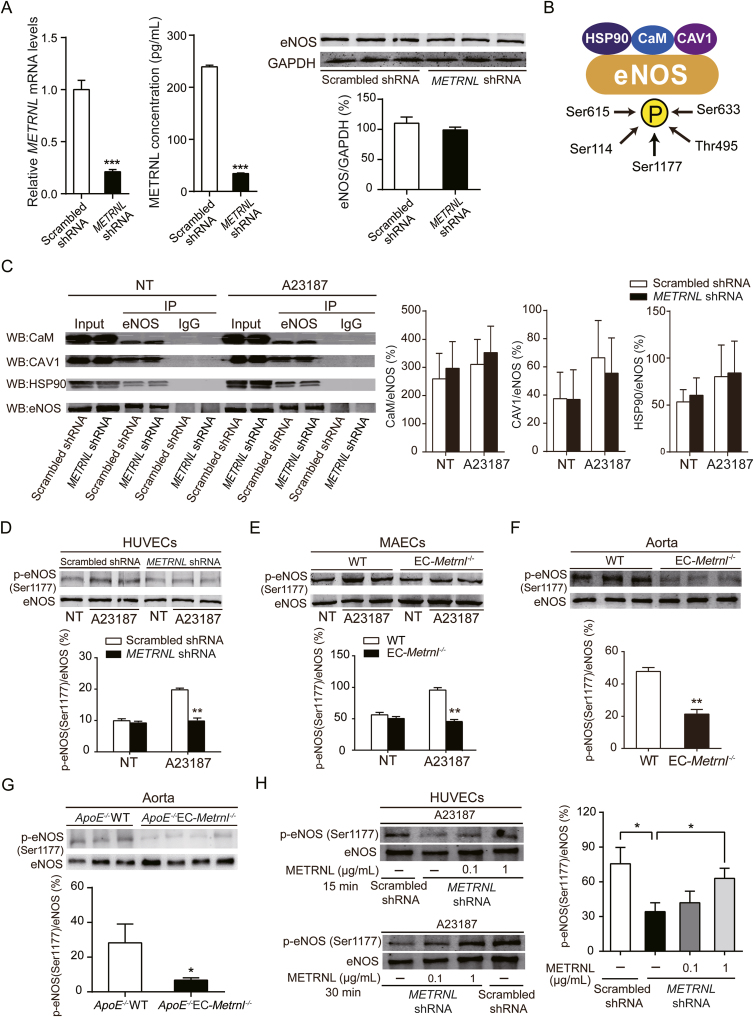
Figure 7.
METRNL deficiency reduces eNOS phosphorylation at Ser1177. (A) eNOS protein level in HUVECs with lentivirus-mediated METRNL knockdown (METRNL shRNA) or vehicle (Scrambled shRNA) (n = 3). (B) Working model of eNOS interacted proteins and eNOS phosphorylations involved in eNOS activity. (C) The interactions between eNOS and calmodulin (CaM), caveolin-1 (CAV1), or heat shock protein 90 (HSP90), detected by immunoprecipitation in HUVECs after METRNL knockdown in presence or absence of eNOS activator (calcium ionophore A23187, 1 μmol/L) during 10 min (n = 3–5). NT, no treatment. (D–H) eNOS phosphorylation at Ser1177 in HUVECs after METRNL knockdown (D), in MAECs (E) and aortae (F) from EC-Metrnl–/– mice and in aortae (G) from ApoE–/–EC-Metrnl–/– mice without atherosclerosis (3 months of age under normal chow). n = 3–4 per group. (H) Exogenous METRNL ameliorates reduced eNOS phosphorylation at Ser1177 in METRNL-deficient HUVECs. Cells were incubated with recombinant METRNL (0.1 or 1 μg/mL) or a solvent control (PBS) for 15 or 30 min, after which A23187 was added at 1 μmol/L for 10 min (n = 3–4). Data are mean ± SEM. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 by two-tailed Student's t test in (A and D–H).