Abstract
Background
Colorectal cancer (CRC) is considered one of the most frequent neoplasms of the digestive tract with a high mortality rate. Left hemicolectomy (LC) and low anterior resection (LAR) with minimally invasive laparoscopic and robotic approaches or with the open technique are the gold standard curative treatment.
Materials and methods
Seventy-seven patients diagnosed with CRC were recruited between September 2017 and September 2021. All patients underwent a preoperative staging with a full-body CT scan. The goal of this study was to compare both types of surgeries, LC-LAR LS with Knight–Griffen colorectal anastomosis and LC-LAR open with Trans-Anal Purse-String Suture Anastomosis (the TAPSSA group), by positioning a No-Coil transanal tube (SapiMed Spa, Alessandria, Italy), in terms of postoperative complications such as prolonged postoperative ileus (PPOI), anastomotic leak (AL), postoperative ileus (POI), and hospital stay.
Results
The patients were divided into two groups: the first with 39 patients who underwent LC and LAR in LS with Knight–Griffen anastomosis (Knight–Griffen group) and the second with 38 patients who underwent LC and LAR by the open technique with the TAPSSA group. Only one patient who underwent the open technique suffered AL. POI was 3.76 ± 1.7 days in the TAPSSA group and 3.07 ± 1.3 days in the Knight–Griffen group. There were no statistically significant differences in terms of AL and POI between the two different groups.
Conclusion
The important point that preliminarily emerged from this retrospective study was that the two different techniques showed similarities in terms of AL and POI, and therefore, all the advantages reported in the previous studies pertaining to No-Coil also hold good in this study regardless of the surgical technique used. However, randomized controlled trials are needed to confirm these findings.
Keywords: colorectal cancer, colorectal anastomosis, complications, no-Coil, surgical oncology
Introduction
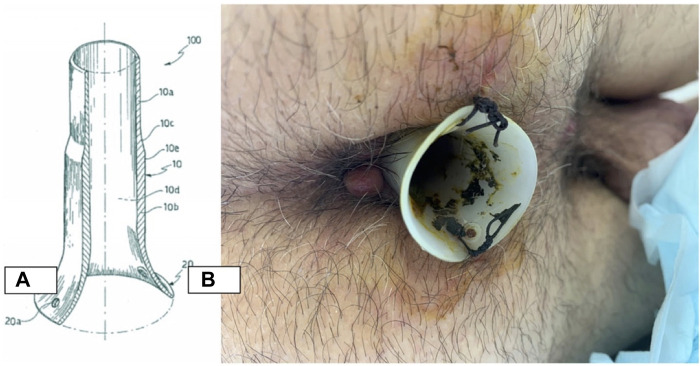
Colorectal cancer (CRC) is considered the most common malignancy in Western countries (1). Accurate preoperative staging is crucial for planning the optimal therapeutic strategy for individual patients. In the preoperative staging of colorectal cancer, computed tomography (CT) is often necessary for devising the best plan for surgery and/or neoadjuvant therapy, particularly when local tumor extension into adjacent organs or distant metastases is detected (2). Low anterior resection (LAR), left hemicolectomy (LC), and right hemicolectomy (RC) remain the treatments of choice, ensuring the best results in terms of quality of life (QoL) and overall survival (Os) (3). It is possible to perform this type of surgery with minimally invasive laparoscopic (LS) and robotic approaches or with the open technique (4). All types of surgeries may cause different complications, and the most frequent of these are prolonged postoperative ileus (PPOI) and anastomotic leak (AL) (5–7). Several factors may contribute to PPOI occurrence, but the level of anastomosis detected is probably the most important one. The parameters used in the literature to describe the incidence rate of AL, regardless of the technicalities surrounding stapled or hand-sewn anastomoses, are at complete variance with the ones used presently, and these depend on the nature of the site in question, that is, a rate of up to 6% for ileo-colic anastomoses, up to 9% for colo-colonic anastomoses, and up to 20% for colo-rectal anastomoses (6). Different studies demonstrate that increased intraluminal rectal pressure is the major contributor to AL (8–11), and for this reason, different endorectal devices (e.g., transanal tube cuff rectum, drainage tube, and silicone transanal tube) have been proposed as promising alternatives to stoma (12, 13). No-Coil® is a transanal silicone stent that allows endorectal decompression, and it is used for the anastomosis of the lower gastrointestinal tract (Figure 1) (14, 15).
Figure 1.
No coil structure and postoperative placement. (A) Length of 60–80 mm, thickness of 2 mm, and diameter of 20 mm. (B) Stabilized 6–8 cm far from the anus through two stitches.
According to recent studies, No-Coil could be considered a useful tool in the prevention of AL-related complications, as it is characterized by ease of use and good feasibility, cost-effectiveness, and favorable patient quality-of-life outcomes after treatment (16). Nevertheless, evidence about No-Coil implementation in the surgical treatment of CRC is limited to a few studies, and definitive conclusions in terms of efficacy cannot be made. Furthermore, these studies examined No-Coil by following the LAR approach, and evidence about its efficacy by following the LC approach is scarce (17).
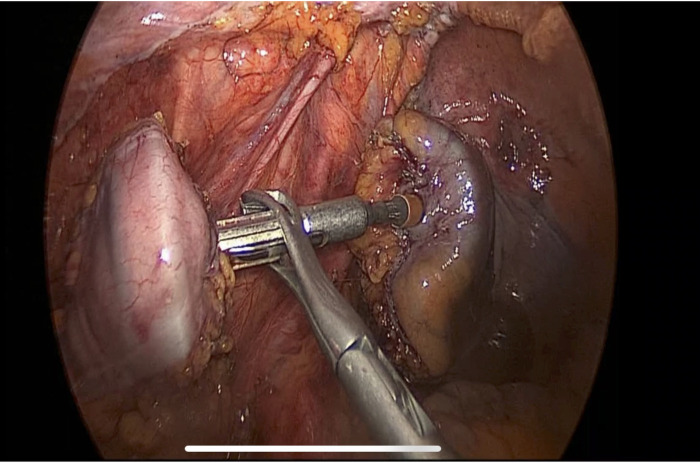
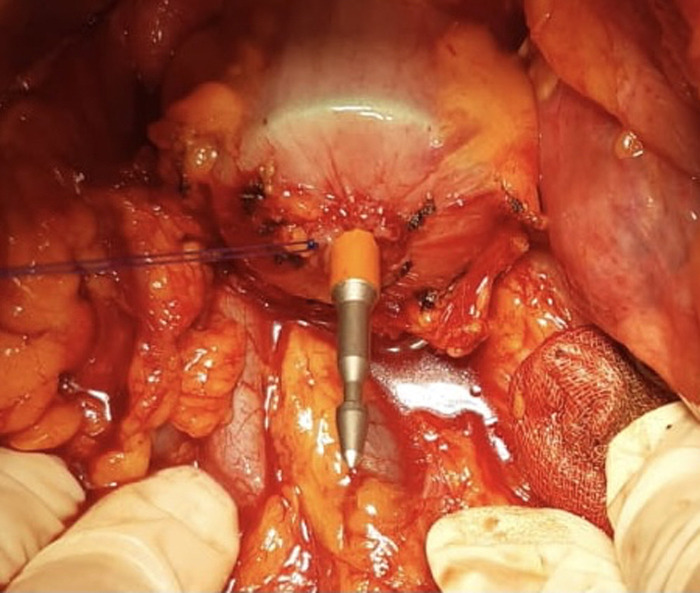
The aim of this study is to improve and analyze the evidence concerning the use of No-Coil and verify its results in two different types of surgeries: LC-LAR LS with Knight–Griffen colorectal anastomosis (Figure 2) and LC-LAR by the open technique with Trans-Anal Purse-String Suture Anastomosis (Figure 3). We analyzed the period of postsurgical hospitalization and the presence of flatus or other complications in all patients who underwent laparoscopic (LS) LAR/LC and open LAR/LC with a No-Coil transanal tube placed at the end of the surgical procedure.
Figure 2.
Knight–Griffen colorectal anastomosis.
Figure 3.
Transanal purse-string suture anastomosis.
Materials and methods
A retrospective study was conducted between September 2017 and September 2021 in which 77 patients diagnosed with a CRC of the descending colon, splenic flexure, sigma, and rectum were recruited at the Digestive Surgery Unit of “Magna Graecia” University Medical School, “Mater Domini” Hospital. LC-LAR LS with Knight–Griffen colorectal anastomosis was performed in 39 patients. Meanwhile, 38 patients underwent LC and LAR by the open technique with Trans-Anal Purse-String Suture Anastomosis (TAPSSA) on Circular Stapler Circular by both techniques measuring 33 mm. All interventions were performed by the same surgeon (MA) in order to reduce possible interoperator errors and distortions and to achieve better accuracy. LC was performed for sigmoid and descending colon tumors, while LAR was applied for the neoplasms of the upper two-thirds of the rectum. Moreover, all patients underwent preoperative staging by total body CT with a three-phase contrast medium so that they could be accurately staged (according to the American Joint of Committee on Cancer 8th Edition) (18).
A possible limitation of this study may stem from the fact that this was a retrospective and non-randomized study, and the patients were assigned to two groups: the first group included patients who received a Knight–Griffen transanal termino-terminal anastomosis in which the section was sutured with a linear suturing machine-type ENDO-GIA and the second group included patients who received a transanal termino-terminal anastomosis in which a tobacco bag was placed on the rectal stump.
The variables analyzed were the duration of the postoperative stays in terms of days, the average time of the first postoperative flatus, and postoperative complications (AL, bleeding, perforation, occlusion, and infections).
In all patients, the surgical technique was standardized with respect to oncological radicality by ligating the artery and mesenteric vein at one centimeter from the origin to ensure a good local staging. Then, standardization was achieved by removing the nearby lymph node chain and freeing the pelvic rectum by performing a total mesorectal excision (TME), which is considered a guideline for rectal tumors.
In this study, the No-Coil tube was placed in all patients after the performance of intestinal anastomosis. It was placed through the sphincter and fixed at a 1–2 cm distance from the anus by making two dots; the tube was removed on the sixth postoperative day if there were no signs of AL. The No-Coil tube, mainly made of silicone, has a length of 60–80 mm, a thickness of 2 mm, and a diameter of 20 mm.
Non-severe surgical complications were corrected by using conservative treatment (Grades I and II according to Clavien Dindo Classification) (19).
Only one exclusion criterion was stipulated in this study: none of the patients should have been administered neoadjuvant therapy. Informed consent was obtained from all patients for performing the study and for the use of medical records. All procedures included in the protocol met the ethical standards of the Helsinki Declaration and the Guidelines for Good Clinical Practice.
Results
Statistical analyses were performed using STATA version 14. Descriptive statistics comprised frequencies and percentages and means and standard deviations. Differences between the case and the control group patients were subsequently explored by using the χ2 test for categorical variables and the T-test for continuous variables. Furthermore, we performed a stepwise linear regression to establish the association between hospital stay (dependent variable), type of surgery, PPOI, AL events (independent variables), and No-Coil placement. All results obtained from the descriptive analysis and comparison between the two groups, called the “Knight–Griffen group” and the “TAPSSA group” (Trans-Anal Purse-String Suture Anastomosis), respectively, for a level of significance of 5% are presented in Table 1.
Table 1.
Analysis and comparison between the two groups.
| TAPSSA (N = 38) | Knight–Griffen (N = 39) | χ2/t | p | |
|---|---|---|---|---|
| Age | 73.6 ± 5.4 | 71.6 ± 7.9 | 19.28 | 0.628 |
| Gender | ||||
| Male | 41 | 0.1225 | 0.122 | |
| Female | 36 | |||
| Hospital stays | 12.02 ± 1.8 | 6.89 ± 1.6 | <0.001 | |
| POI | 3.76 ± 1.7 | 3.07 ± 1.3 | 20.670 | 0.342 |
| PPOI | 9 (23.6%) | 1 (2.56%) | 7.597 | 0.006 |
| AL | 1 (2.63%) | 0 | 1.039 | 0.308 |
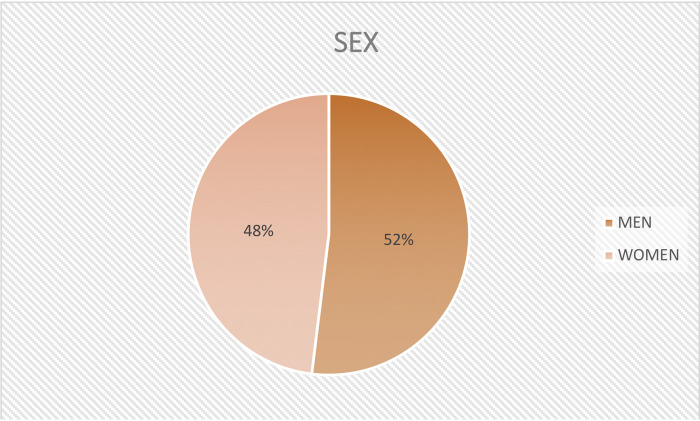
No differences emerged in terms of gender (χ2 = 0.1225; p = 0.726) and age (χ2 = 19.3; p = 0.628) distribution between the two groups (Figure 4).
Figure 4.
Percentage of men and women in the study.
LC and LAR in LS were performed in 39 patients (50.65%) and LC and LAR by the open technique was performed in 38 patients (49.35%).
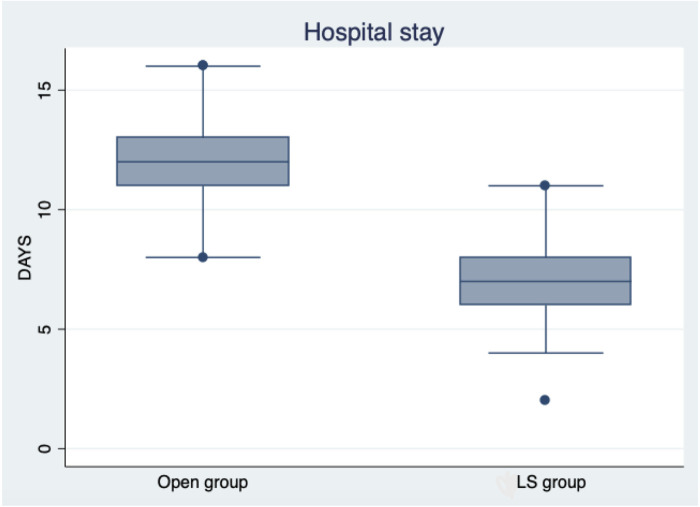
No postoperative incontinence or constipation was reported in the patients. Mean hospital stay was 7 ± 1.6 days in LS group patients and 12 ± 1.8 in open group ones (Figure 5). AL was present in one patient (2.63%) in the open surgery group and in none in the LS group; AL in one patient was treated with the conservative method with total parenteral nutrition and removal of the No-Coil transanal tube on the 12th day. No statistical difference was detected in AL appearance between the groups (χ2 = 1.03; p = 0.308). PPOI was not associated with any independent variables. AL was not associated with POI days (log-likelihood = 5.0702; LR χ2 = 0.53; Prob > χ2 = 0.464; Pseudo R2 = 0.050;95% CI = 6.5269–14.271; p = 0.528) and also with PPOI (log-likelihood = 25.053; LR χ2 = 9.36; Prob > χ2 = 0.464; Pseudo R2 = 0.022; 95% CI = 2.266–3.227; p = 0.408).
Figure 5.
Box plot shows the difference between the open group and the LS group in terms of hospital stay.
A comparison of the results of the two groups showed that No-Coil placement reduced the postoperative complications of AL and POI with no differences between the two surgical techniques. But in terms of PPOI and hospital stay, the LS approach with Knight–Griffen anastomoses was found to be better than the traditional open methods with TAPSSA (Table 1).
Discussion
The purpose of this retrospective study was to examine the differences between two types of surgery in terms of postoperative complications, AL, PPOI, POI, and hospital stay with the aid of the No-Coil® device. For the staging of the disease, particularly for the detection of liver and extrahepatic metastasis, we performed a full-body CT scan, which, according to different studies, is considered the first-choice procedure and the best imaging examination in terms of cost-effectiveness (20, 21). In this study, only one out of a total of 77 patients suffered AL. Hence, no differences were detected on the basis of the use of No-Coil after the performance of the surgery. Only a few studies have explored the efficacy of No-Coil use in reducing this type of leak. In one of them, Montemurro et al. evaluated AL prevalence in a sample of 184 patients undergoing elective total or subtotal proctectomy with low-lying anastomosis and found an AL incidence rate of almost 4.8%, which was higher than that of this study (14). Particularly, two randomized trials considered the use of a transanal stent other than No-Coil structurally different from it. Sciuto et al., in their study, analyzed the predictive factors of anastomotic losses after laparoscopic colorectal surgery, and there were several studies between 2008 and 2018 on laparoscopic colorectal procedures with left anastomosis (7).
In these studies, the incidence rates ranged from 78% (Lee et al.) in 2017 to 50% (Van Praagh et al.) in 2016 with an average of 11.1% (22, 23).
Considering all the above factors and that in our study we had only one patient with AL, which may be attributed to the placement of No-Coil, further follow-up studies are needed so that more insights can be had about AL (15). Amin et al. analyzed the occurrence of AL following the placement of an LAR plus transanal stent, as opposed to Tau protein (defunctioning stoma), and found anastomotic leakage in 3 of 41 patients (approximately 7%) (9). In contrast, Bulow et al. found that the transanal stent was not superior to a defunctioning stoma in preventing the risk of AL after LAR (about 10.7%) (11). Chen et al., who studied 1,262 patients without, and 1,170 patients with, a transanal drainage tube after laparoscopic anterior resection for rectal cancer concluded that placement was associated with significantly lower rates of AL and reoperation, and hence, it was likely to be an effective method of preventing and reducing AL after rectal cancer surgery (24). Zhao et al., in 576 consecutive patients, indicated that the transanal drainage tube may not confer any benefit for AL prevention in patients who undergo laparoscopic low anterior resection for mid-low rectal cancer without preoperative radiotherapy (25).
Although contrary opinions are expressed in studies in the literature, almost all focus on a single technique and complication using different types of devices. These study results cannot be compared with the present results because the efficacy of different devices varies depending on the different purposes for which they are used. The device used by us is also a unique and non-fungible one, and the results obtained by us are related more to postoperative complications (AL, PPOI, POI, hospital stay) and two different patient groups that were compared, in which two different techniques were used, the Knight–Griffen group with the LS approach vs. the TAPSSA group with the traditional open method.
Our data are preliminary in nature and may have prevented us in finding the significance between the two different techniques in terms of AL and POI postoperative complications. For these reasons, other studies are necessary to confirm the evidence presented. The mean age of the patients of the groups was approximately 70 years and not lower.
This study confirmed, as shown in the literature, that laparoscopic intervention is characterized by a lower rate of PPOI than open surgery. In 13 RCTs instead, defining PPOI as a “reintegration of SNG”, the incidence rate of PPOI was 14.2% (95% CI 7.2%–26%) for colon or rectum resections and 30.9% (95% CI 12.7%–57.8%) for rectal resections. After laparoscopic resections, the incidence rate of PPOI was lower, approximately 6.4% (95% CI 3.5%–11.5%), but 10% (95% CI 6.2%–15.8%) after open colorectal resection (26, 27). Logically, it is not possible to compare these data with those of our study, as the definitions of PPOI, the number and characteristics of patients, and the type of intervention are not unambiguous in nature. Nevertheless, the fact that it was never necessary to reintroduce the SNG and that on the fifth postoperative day the incidence of PPOI was low suggests that the use of No-Coil has a positive influence on PPOI.
Conclusion
According to the data collected on 77 patients, even in such a preliminary type of study, we can reasonably assume that with the positioning of the No-Coil tube, we were able to reduce the time of canalization to gases and feces and the risk of AL in both groups. Furthermore, by associating the No-Coil placement with the LS approach, we can reduce a number of postoperative complications related to PPOI and hospital stay. Another great advantage relating to the quality of life of the patients, their psychological health, and the costs for the National Health System stems from the elimination of the need to perform protective ileostomy and the subsequent stoma closure surgery. Also, we observed that the use of No-Coil allowed the patients to achieve faster mobility, given the high level of importance attached to the performance of these major surgical procedures.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The Human Investigation Committee (IRB) of University “Magna Graecia” Medical School, “Mater Domini” Hospital, approved this retrospective study (Protocol N° 182, 18 June 2020). The patients/participants provided their written informed consent to participate in this study.
Author contributions
MA, GC, and SM conceived the study design. FF, RoM, RiM, and RR were involved in data collection and drafting of the work. DL, FM, NA, and GN revised the work critically. MA and CB performed statistical analysis and revision and GC gave the final approval. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392(10159):1736–88. 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin SS, Jeong YY, Min JJ, Kim HR, Chung TW, Kang HK. Preoperative staging of colorectal cancer: CT vs. Integrated FDG PET/CT. Abdom Imaging. (2008) 33(3):270–7. 10.1007/s00261-007-9262-9 [DOI] [PubMed] [Google Scholar]
- 3.McLeod RS. Comparison of quality of life in patients undergoing abdominoperineal extirpation or anterior resection for rectal cancer. Ann Surg. (2001) 233(2):157–8. 10.1097/00000658-200102000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiappa A, Biffi R, Zbar AP, Bertani E, Luca F, Pace U, et al. The influence of type of operation for distal rectal cancer: survival, outcomes, and recurrence. Hepatogastroenterology. (2007) 54(74):400–6. PMID: [PubMed] [Google Scholar]
- 5.Han KS, Choi GS, Park JS, Kim HJ, Park SY, Jun SH. Short-term outcomes of a laparoscopic left hemicolectomy for descending colon cancer: retrospective comparison with an open left hemicolectomy. J Korean Soc Coloproctol. (2010) 26(5):347. 10.3393/jksc.2010.26.5.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An V, Chandra R, Lawrence M. Anastomotic failure in colorectal surgery: where are we at? Indian J Surg. (2018) 80(2):163–70. 10.1007/s12262-018-1745-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sciuto A, Merola G, Palma GDD, Sodo M, Pirozzi F, Bracale UM, et al. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. WJG. (2018) 24(21):2247–60. 10.3748/wjg.v24.i21.2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mander BJ, Wexner SD, Williams NS, Bartolo DC, Lubowski DZ, Oresland T, et al. Preliminary results of a multicentre trial of the electrically stimulated gracilis neoanal sphincter. Br J Surg. (1999) 86(12):1543–8. 10.1046/j.1365-2168.1999.01285.x [DOI] [PubMed] [Google Scholar]
- 9.Sterk P, Schubert F, Günter S, Klein P. Anastomotic protection with a transanal tube after rectum resection and total mesorectal excision. Zentralbl Chir. (2001) 126(8):601–4. 10.1055/s-2001-16569 [DOI] [PubMed] [Google Scholar]
- 10.Amin AI, Ramalingam T, Sexton R, Heald RJ, Leppington-Clarke A, Moran BJ. Comparison of transanal stent with defunctioning stoma in low anterior resection for rectal cancer. Br J Surg. (2003) 90(5):581–2. 10.1002/bjs.4074 [DOI] [PubMed] [Google Scholar]
- 11.Bülow S, Bulut O, Christensen IJ, Harling H, Rectal Stent Study Group. Transanal stent in anterior resection does not prevent anastomotic leakage. Colorectal Dis. (2006) 8(6):494–6. 10.1111/j.1463-1318.2006.00994.x [DOI] [PubMed] [Google Scholar]
- 12.Brandl A, Czipin S, Mittermair R, Weiss S, Pratschke J, Kafka-Ritsch R. Transanal drainage tube reduces rate and severity of anastomotic leakage in patients with colorectal anastomosis: a case controlled study. Ann Med Surg (Lond). (2016) 6:12–6. 10.1016/j.amsu.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye W, Zhu Z, Liu G, Chen B, Zeng J, Gao J, et al. Application of the cuff rectum drainage tube in total mesorectal excision for low rectal cancer: a retrospective case- controlled study. Medicine (Baltimore). (2019) 98(23):e15939. 10.1097/MD.0000000000015939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montemurro S, De Luca R, Caliandro C, Ruggieri E, Rucci A, Sciscio V, et al. Transanal tube NO COIL® after rectal cancer proctectomy. The “G. Paolo II” Cancer Centre experience. Tumori. (2012) 98(5):607–14. 10.1177/030089161209800511 [DOI] [PubMed] [Google Scholar]
- 15.Ammendola M, Ruggiero M, Talarico C, Memeo R, Ammerata G, Capomolla A, et al. No coil® placement in patients undergoing left hemicolectomy and low anterior resection for colorectal cancer. World J Surg Onc. (2020) 18(1):327. 10.1186/s12957-020-02096-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zawadzki M, Krzystek-Korpacka M, Rząca M, Czarnecki R, Obuszko Z, Sitarska M, et al. Risk factors in reoperations in colorectal surgery. Pol Przegl Chir. (2019) 91(4):13–8. 10.5604/01.3001.0013.1922 [DOI] [PubMed] [Google Scholar]
- 17.Montemurro S, Ammendola M, Gallo G, Romano R, Condoluci A, Curto L, et al. Sphincter-saving proctectomy for rectal cancer with NO COIL® transanal tube and without ostoma. Clinical outcomes, cost effectiveness and quality of life in the elderly. Minerva Chir. (2019) 74(1):19–25. 10.23736/S0026-4733.18.07755-6 [DOI] [PubMed] [Google Scholar]
- 18.Weiser MR. Colorectal cancer. Ann Surg Oncol (AJCC 8th Edition). (2018) 25:1454–5. 10.1245/s10434-018-6462-1 [DOI] [PubMed] [Google Scholar]
- 19.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250(2):187–96. 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 20.Bipat S, Niekel MC, Comans EFI, Nio CY, Bemelman WA, Verhoef C, et al. Imaging modalities for the staging of patients with colorectal cancer. Neth J Med. (2012) 70(1):26–34. PMID: [PubMed] [Google Scholar]
- 21.Bipat S, van Leeuwen MS, Comans EFI, Pijl MEJ, Bossuyt PMM, Zwinderman AH, et al. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis—meta-analysis. Radiology. (2005) 237(1):123–31. 10.1148/radiol.2371042060 [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Ahn B, Lee S. The relationship between the number of intersections of staple lines and anastomotic leakage after the use of a double stapling technique in laparoscopic colorectal surgery. Surg Laparosc Endosc Percutan Tech. (2017) 27(4):273–81. 10.1097/SLE.0000000000000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Praagh JB, de Goffau MC, Bakker IS, van Goor H, Harmsen HJM, Olinga P, et al. Mucus microbiome of anastomotic tissue during surgery has predictive value for colorectal anastomotic leakage. Ann Surg. (2019) 269(5):911–6. 10.1097/SLA.0000000000002651 [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Cai HK, Tang YH. An updated meta-analysis of transanal drainage tube for prevention of anastomotic leak in anterior resection for rectal cancer. Surg Oncol. (2018) 27(3):333–40. 10.1016/j.suronc.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 25.Zhao S, Zhang L, Gao F, Wu M, Zheng J, Bai L, et al. Transanal drainage tube use for preventing anastomotic leakage after laparoscopic low anterior resection in patients with rectal cancer: a randomized clinical trial. JAMA Surg. (2021) 156(12):1151–8. 10.1001/jamasurg.2021.4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolthuis AM, Bislenghi G, Fieuws S, D’Hoore A. Incidence of prolonged postoperative ileus after colorectal surgery: a systematic review and meta-analysis. Colorectal Dis. (2016) 18(1):O1–9. 10.1111/codi.13210 [DOI] [PubMed] [Google Scholar]
- 27.Ammendola M, Ammerata G, Filice F, Filippo R, Ruggiero M, Romano R, et al. Anastomotic leak rate and prolonged postoperative paralytic ileus in patients undergoing laparoscopic surgery for colo-rectal cancer after placement of No-coil endoanal tube. Surg Innov. (2022) 30(1):20–7. doi: 10.1177/15533506221090995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.