PURPOSE
Both the performance characteristics of prostate-specific membrane antigen positron emission tomography and insurance approval improves with increasing prostate-specific antigen (PSA) level causing some physicians to delay post-radical prostatectomy salvage radiation therapy (sRT) after PSA failure. Yet, it is unknown for men with at most one high-risk factor (ie, pT3/4 or prostatectomy [p] Gleason score 8-10) whether a PSA level exists above which initiating sRT is associated with increased all-cause mortality (ACM)-risk and was investigated.
METHODS
Using a multinational database of 25,551 patients with pT2-4N0 or NXM0 prostate cancer, multivariable Cox regression analysis evaluated whether an association with a significant increase in ACM-risk existed when sRT was delivered above a prespecified PSA level beginning at 0.10 ng/mL and in 0.05 increments up to 0.50 ng/mL versus at or below that level. The model was adjusted for age at and year of radical prostatectomy, established prostate cancer prognostic factors, institution, and the time-dependent use of androgen deprivation therapy.
RESULTS
After a median follow-up of 6.00 years, patients who received sRT at a PSA level >0.25 ng/mL had a significantly higher ACM-risk (AHR, 1.49; 95% CI, 1.11 to 2.00; P = .008) compared with men who received sRT when the PSA was ≤0.25 mg/mL. This elevated ACM-risk remained significant for all PSA cutpoints up to 0.50 ng/mL but was not significant at PSA cutpoint values below 0.25 ng/mL.
CONCLUSION
Among patients with at most one high-risk factor, initiating sRT above a PSA level of 0.25 ng/mL was associated with increased ACM-risk.
INTRODUCTION
Prostate-specific membrane antigen positron emission tomography (PSMA-PET) as compared with conventional imaging (bone scan, computed tomography, or magnetic resonance imaging of the abdomen and pelvis) seems to improve the detection of clinical recurrence in men with prostate-specific antigen (PSA) failure and a PSA level of at least 0.20 ng/mL after radical prostatectomy (RP) for prostate cancer (PC)1 and is US Food and Drug Administration approved. The approval was supported by findings from the phase III CONDOR trial,1 in which 63.9% of men with PSA failure (≥0.20 ng/mL) who did not have definitive evidence of recurrence using standard imaging had a change in management on the basis of the 18F-DCFPyL-PET/CT findings. The correct localization rate (CLR) on the basis of histopathology, subsequent confirmatory imaging, or post-RT PSA response of PSMA PET/CT according to PSA levels ranged from 73%-77%, 73%-77%, 78%-85%, 80%-92%, and 90%-97% for men whose PSA level ranged from <0.5, 0.5-1.0, 1.0-2.0, 2.0-5.0, or >5.0 ng/mL, respectively. Yet, at lower PSA levels <0.2 ng/mL, a meta-analysis2 where only histopathology was used to confirm the CLR found this value to be only 40% using 68Ga-PSMA-11 PET. We would expect a similar CLR using the 18F-PSMA-11 tracer given that in a prospective double blinded randomized cross over study design the tracer 18F-PSMA-11 was found to be noninferior to 68Ga-PSMA-11 in detecting PC in men with newly diagnosed or biochemically recurrent PC after RP.3 With only a 40% chance of correctly identifying recurrent disease at PSA levels <0.2 ng/mL, many insurers in the United States (eg, Blue Cross and Blue Shield) will not reimburse a PSMA-PET scan unless the patient has documented PSA failure (≥0.20 ng/mL and rising) as per the American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology definition.4,5
CONTEXT
Key Objective
Can delaying salvage radiation therapy after radical prostatectomy for prostate cancer in men with one high-risk factor (prostatectomy [p] T3/4 or pGleason score 8-10) to obtain a prostate-specific membrane antigen positron emission tomography scan that will be covered by insurers and also have a higher positive predictive value lead to an increased risk of death?
Knowledge Generated
Waiting to deliver salvage radiation therapy up until a prostate-specific antigen (PSA) level of 0.25 ng/mL was not associated with an increased risk of all-cause mortality; however, this was not true for PSA levels above 0.25 ng/mL.
Relevance (M. Carducci)
Initiating salvage radiotherapy postprostatectomy before the PSA exceeds 0.25 ng/mL is made clearer in this report. With wider use of ultrasensitive PSA in high-risk individuals, individuals can move to salvage therapies before novel imaging can identify sites of persistent disease.*
*Relevance section written by JCO Associate Editor Michael Carducci, MD.
Given that both the performance characteristics of PSMA-PET and insurance approval improve with increasing PSA level, some physicians choose not to initiate post-RP salvage radiation therapy (sRT) until the PSA level exceeds 0.20 ng/mL. Yet, it is unknown whether a PSA level exists above which initiating sRT is associated with an increased all-cause mortality (ACM)-risk and was investigated.
METHODS
Patient Population and Treatment
The study cohort comprised 25,551 patients of median age 64 (interquartile range [IQR], [59 to 79]) years with prostatectomy (p) T2-4N0 or NXM0 PC consecutively treated between June 15, 1990, and June 19, 2020, with RP and pelvic lymph node assessment when appropriate at the University Hospital Hamburg-Eppendorf (Hamburg, Germany, N = 24,345) or the University of California, San Francisco (UCSF, N = 1,206). Approximately 1% and 17% percent of the patients were from underrepresented backgrounds in the Hamburg and UCSF cohorts, respectively, where both public and private insurance was honored as well as free care for those with no insurance at UCSF. Patients from Germany and California as well other parts of Europe and the United States were represented in the study cohort. Patients with two high-risk features (ie, pGleason score 8-10 and pT3 or pT4) where the use of adjuvant (a) (ie, generally delivered with 6 months of RP when the PSA level is undetectable) as compared with early sRT has been shown to be associated with decreased ACM-risk6 were excluded as were patients with a persistent PSA after RP. Therefore, patients included in this study could have at most one high-risk factor (ie, pGleason score 8-10 or pT3 or pT4) and needed to have achieved an undetectable PSA after RP. The distribution of the time-dependent use after RP (time 0) of adjuvant RT (aRT) and early sRT when the PSA level was >0.25 ng/mL or ≤0.25 ng/mL is illustrated in the flow diagram as shown in Figure 1. aRT and sRT to the pelvic LNs (45 Gy [Gy]) when felt appropriate by the treating physician and prostatic bed (median dose: 68.4 Gy) were delivered at a median of 3.55 (IQR, 2.96-4.21) months and 25.89 (IQR, 12.25-48.49) months, respectively, after RP.
FIG 1.
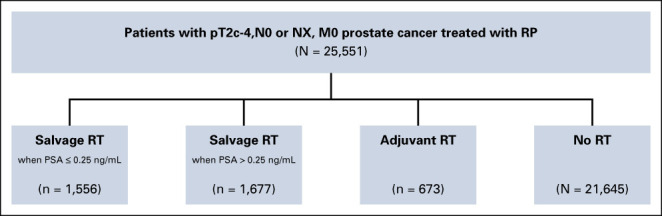
Flow diagram illustrating the distribution of no RT, adjuvant RT, salvage RT when the PSA ≤0.25 ng/mL or >0.25 ng/mL after RP among the 25,511 patients in the study cohort. Given time 0 is defined as the date of RP, the numbers of men who in the no RT, adjuvant RT, and salvage RT cohorts are time dependent and represent the values at last follow-up. aRT, adjuvant RT; ng/mL; nanograms/milliliter; PSA, prostate-specific antigen; RP, radical prostatectomy; RT, radiation therapy.
Prostatectomy and lymph node specimens underwent review by a pathologist with expertise in genitourinary pathology. In accord with federal and institutional guidelines, men signed an institutional review board–approved, protocol-specific informed consent form permitting prospective collection of deidentified data at baseline and follow-up, which were entered into a secure, password-protected database for outcome analysis. A minority of the data were collected retrospectively.
Follow-Up and Determination of the Cause of Death
Follow-up started on the day of RP and concluded on the date of last follow-up or the date of death, whichever came first. The database was last updated on June 23, 2022. Other than death, no patient was lost to follow-up. During follow-up, patients had a PSA test and rectal examination and were seen every 3 months for 1 year, every 6 months for an additional 4 years, and then annually thereafter. Salvage ADT was delivered after PSA failure and clinical or radiographic evidence of progression after receiving aRT or sRT. At the time of progression to castrate-resistant M0 or M1 disease, the practice patterns followed the treatment guidelines set forth by the American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology4,5 or European Association of Urology.7 To assign PC-specific mortality (PCSM) as the cause of death, castrate-resistant metastatic PC on the basis of a rising PSA level in the setting of a testosterone level <20 ng/dL before death needed to be confirmed and in addition the treating oncologist or urologist at the time of death needed to assign PC as the primary cause of death and record this on the death certificate.
Statistical Methods
Comparison of the distribution of the patient characteristics at the time of RP stratified by postoperative treatment.
Comparisons of the distribution of the patient characteristics at the time of RP across the four time-dependent treatment groups (no RT, aRT, sRT delivered when the PSA level was >0.25, and sRT delivered when the PSA level was ≤0.25 ng/mL [baseline]) were made using a Mantel Haenszel Chi-Square metric8 for categorical variables. In the case of a small sample size, the Fisher exact test9 was used. For continuous variables such as age at and year of RP, medians and their distributions were compared using a Wilcoxon two-sample test.10
Univariable and multivariable hazard ratios for ACM-risk.
Cox regression univariable and multivariable analyses11 were used to evaluate whether there was an association with a significant increase in ACM-risk when sRT was delivered above a prespecified PSA level including 0.10, 0.15 ng/mL… up to 0.50 ng/mL in 0.05 ng/mL increments versus at or lower than PSA level adjusting for age at and year of RP, established PC prognostic factors, institution with University Hospital Hamburg-Eppendorf as the baseline institution, and the time-dependent12 use of ADT. Other time-dependent treatment groups included in model were no RT (ie, men who never progressed or who were treated with sADT alone at progression), aRT, and sRT delivered when the PSA was > versus ≤ than the prespecified PSA cutpoint. sRT delivered when the PSA was ≤ than the prespecified PSA cutpoint served as the baseline treatment group. The date of RP was defined as time 0. ADT could be delivered in the adjuvant or salvage setting and was treated as a time-dependent covariate,12 age at and year of RP which were treated as continuous covariates, and the established prognostic factors of the pre-RP PSA level (4-10 ng/mL [baseline], <4 ng/mL, >10 ng/mL] pGleason score [6 (baseline), 7, 8-10] and margin status (positive v negative [baseline]) were treated as categorical covariates. ACM unadjusted and adjusted hazard ratios (AHRs) are reported with associated 95% CI for all covariates. At the PSA level where ACM-risk was significantly increased when sRT was initiated above as compared with at or below that level, we performed a Fine and Grays Regression competing risk multivariable regression analysis13 evaluating the end point of PCSM using the same covariates for adjustment as used in the Cox model11 evaluating ACM-risk.
Adjusted estimates of ACM.
For the purpose of illustration, adjusted estimates of ACM (1-minus Kaplan-Meier estimate14 of overall survival) were calculated for each of the four post-RP time-dependent treatment groups. These estimates among patients who received treatment with sRT delivered when the PSA level was >0.25 ng/mL, aRT, and no RT were compared with the baseline treatment group of sRT delivered when the PSA level was ≤0.25 ng/mL. ACM estimates were adjusted for established prognostic factors, age15 at and year of RP, and the time-dependent use12 of ADT. A two-sided P value ≤.05 was considered statistically significant, and the Bonferroni method16 was used for multiplicity adjustment of the three comparisons such that the P value needed to be ≤.05/3 or ≤.0167 to be considered significant. P values for the adjusted plots were calculated using the Cox11 model and were adjusted for both fixed and time-dependent covariates. R (version 4.2.1; R Foundation for statistical computing) was used to calculate Kaplan-Meier estimates with time-dependent treatment and ADT use covariates. SAS (version 9.4; SAS institute Inc) was used for all other calculations.
RESULTS
Comparison of the Distribution of the Patient Characteristics at the Time of RP Stratified by Postoperative Treatment
Among the 25,551 patients, 1,556 (6.09%) underwent sRT when the PSA level was ≤0.25 ng/mL, whereas 1,677 (6.56%) underwent sRT when the PSA level was >0.25 ng/mL. aRT was delivered to 673 (2.63%) patients and 206 (0.81%) patients underwent aADT for a median duration of 6.01 (IQR, 2.92-10.28) months. Salvage ADT was delivered to 1,489 (5.83%) men. As shown in Table 1, among patients who received sRT at a PSA level >0.25 ng/mL as compared with ≤0.25 ng/mL, there was a significantly higher proportion of patients with adverse pathologic and clinical factors (all P values ≤ .005) including pT3b/4, pre-RP >10 ng/mL, and an increased use of sADT (39.65% v 27.44%, P < .001).
TABLE 1.
Comparison of the Distribution of the Patient Characteristics at the Time of RP Stratified by Whether They Received sRT at a PSA Value Not Exceeding 0.25 ng/mL or at a Value >0.25 ng/mL, Adjuvant or No Radiation Therapy
Univariable and Multivariable Hazard Ratios for ACM-Risk
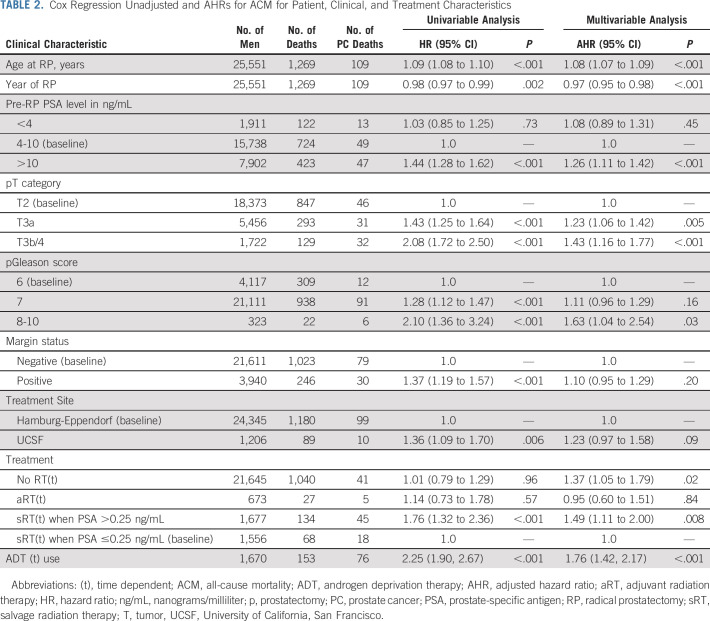
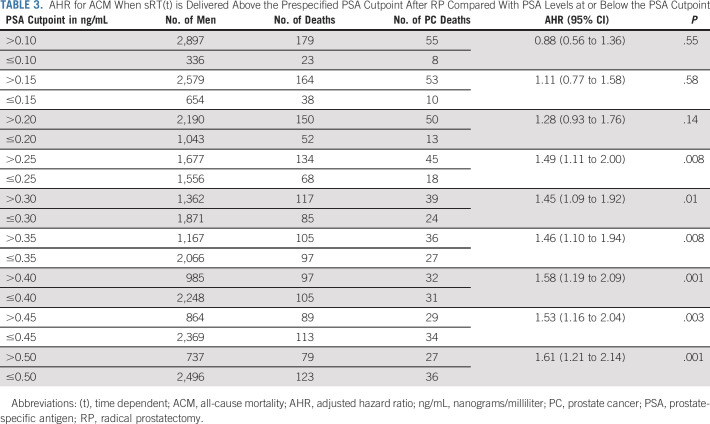
After a median follow-up of 6.00 years (IQR, 3.01-9.15), 1,269 men died, 109 (8.59%) from PC. Patients who received sRT at a PSA level >0.25 ng/mL had a significantly higher ACM-risk (AHR, 1.49; 95% CI, 1.11 to 2.00; P = .008) compared with men who received sRT when the PSA was ≤0.25 mg/mL as shown in Table 2. Similarly, an elevated risk of PCSM (AHR, 1.43; 95% CI, 0.80 to 2.55) was observed in men whose sRT was initiated at a PSA level >0.25 ng/mL compared with 0.25 ng/mL or less. The elevated ACM-risk remained significant for all PSA cutpoints above 0.25 ng/mL with an AHR of 1.61 [1.21, 2.14]; P = .001 at a PSA cutpoint of 0.50 ng/mL but was not significant at PSA cutpoint values below 0.25 ng/mL as shown in Table 3. There was no significant difference in ACM-risk when comparing aRT use to sRT delivered when the PSA level was ≤0.25 ng/mL (AHR, 0.95; 95% CI, 0.60 to 1.51; P = .84).
TABLE 2.
Cox Regression Unadjusted and AHRs for ACM for Patient, Clinical, and Treatment Characteristics
TABLE 3.
AHR for ACM When sRT(t) is Delivered Above the Prespecified PSA Cutpoint After RP Compared With PSA Levels at or Below the PSA Cutpoint
Adjusted Estimates of ACM
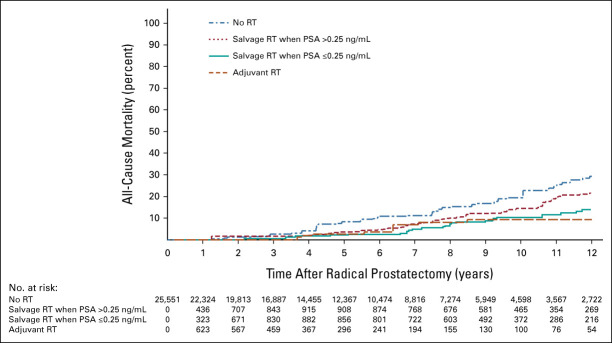
As shown in Figure 2, the 10-year adjusted point estimates for ACM were significantly higher among patients who received no RT (P = .01) or sRT when the PSA was >0.25 ng/mL (P = .008) but not aRT (P = .78) compared with patients who received sRT when the PSA level was ≤0.25 ng/mL. At 10 years, these respective ACM point estimates (95% CI) were 19.44% (13.72%, 27.15%), 14.48% (10.17%, 20.39%), 9.29% (4.07%, 20.46%), and 10.36% (6.26%, 16.01%), respectively.
FIG 2.
Adjusted estimates of ACM among men illustrating the difference in these estimated among patients in the time-dependent treatment groups of no RT, aRT, salvage RT when the PSA level >0.25 ng/mL compared with salvage RT when the PSA level is ≤0.25 ng/mL after RP (baseline). ACM, all-cause mortality; aRT, adjuvant RT; ng/mL, nanograms/milliliter; PSA, prostate-specific antigen; RP, radical prostatectomy; RT, radiation therapy.
DISCUSSION
In this study, we found that initiating sRT after RP when the PSA level exceeded 0.25 ng/mL as opposed to earlier was associated with a significant increase in ACM-risk. The clinical relevance of this finding is that some physicians are waiting until the PSA level exceeds 0.25 ng/mL in the post-RP setting to obtain a PSMA-PET scan and then initiate salvage treatment for two reasons. First, the performance characteristics of PSMA-PET improves with increasing PSA levels1,2 thus minimizing false-positive and false-negative findings. Second, many insurers will not reimburse a PSMA PET scan until the PSA level exceeds a prespecified PSA threshold that is insurance specific. The results of the current study provide evidence to support that by waiting to initiate sRT after PSA failure may place some patients at increased ACM-risk.
Several points deserve further consideration. First, although PSMA-PET has been shown to change the RT management of men in post-RP salvage setting,1 prospectively acquired randomized evidence as to whether these changes in management affect cancer control outcomes is not yet available but is being addressed in a prospective randomized controlled trial (RCT).17 The specific question being addressed in that RCT is whether information provided by the PSMA-PET versus conventional imaging post-RP and obtained when the PSA level is >0.10 ng/mL can improve PSA failure-free survival at 5 years because of changes in management based solely on the PSMA-PET findings. This study is important because the random assignment should provide balance in both known and unknown prognostic factors across imaging arms, and moreover, the results will enable us to discern whether management changes on the basis of the PSMA-PET scan affect a relevant cancer control end point. Second, in the current study in addition to adjusting for age and known PC prognostic factors, we also adjusted for the time-dependent use and duration of ADT. This is particularly important given that the use of ADT and its duration in the post-RP setting has been shown in two prospective randomized trials18,19 to be associated with the reduction of metastasis-free survival and in addition prolongation in overall survival in one study.20 Third, during the conduct of the study, there were advances in the postoperative PSA assay to ultrasensitive, variability in Gleason Score assignment even among experienced pathologists and improvements in survival in patients with PC. Therefore, we added the year of RP as a covariate to the model to adjust for changes that happened over the study period, at least in part, if not fully such as improved survival in patients with PC due to treatment advances and/or biologic evolution as well as reclassification of the Gleason scores from 6 to 7 or higher after the changes in the Gleason grading system adopted in 2005 by the International Society of Urologic Pathology.21 These factors would lead to a reduced risk of ACM later in the study as compared with earlier, and this is reflected in the adjustment for increasing year of RP in the model where the AHR of 0.97 was significant reflecting a 3% reduction in ACM-risk with each advancing year when the RP was performed. Although the use of the ultrasensitive PSA after RP began during the conduct of the study, our finding of an increased ACM-risk when initiating sRT at a PSA cutpoint >0.25 ng/mL versus 0.25 ng/mL or less should not have been affected by the ultrasensitive PSA assay that measure PSA levels as low as 0.01 ng/mL given that the PSA levels that were measurable at the start of our study were 0.20 ng/mL or higher. Fourth, in the Radiotherapy and Androgen deprivation after local surgery-RT randomized trial22 which could not establish superiority of adjuvant to early post-RP sRT with respect to disease-free survival, the median (IQR) PSA level at the start of early sRT was 0.20 ng/mL [0.10-0.30] meaning that 50% of the men enrolled on that study had sRT initiated at a PSA level ≤0.20 ng/mL. Our results are consistent with this finding in that in our patient population where men could have at most one high-risk factor (ie, pGleason score 8-10 or pT3/4), we found no significant difference in ACM-risk for the use of aRT compared with sRT delivered when the PSA level was ≤0.25 ng/mL. Finally, although only a prospective RCT enrolling patients with at least one high-risk factor and randomly assigning them to initiating sRT when the PSA level after RP is >0.25 ng/mL versus ≤0.25 ng/mL can establish causality between delivering sRT when the PSA level is >0.25 ng/mL and an increased ACM-risk, such a trial is currently not ongoing or planned.
Therefore, the data in the current study provide the only available evidence to support initiating sRT after RP at a PSA level that is ≤0.25 ng/mL. This observation is clinically significant given that delivering sRT at PSA levels exceeding 0.25 ng/mL is associated with a higher ACM-risk.
Derya Tilki
Honoraria: Janssen, Ipsen, Exact Sciences, Apogepha, AstraZeneca, Advanced Accelerator Applications, Roche, Takeda, miR Scientific
Consulting or Advisory Role: miR Scientific, AstraZeneca, Roche
Research Funding: Janssen
Ming-Hui Chen
Employment: Boehringer Ingelheim
Markus Graefen
Honoraria: Astellas Pharma, Bayer, Takeda, Janssen, Medtronic
Consulting or Advisory Role: Medtronic
Travel, Accommodations, Expenses: Astellas Pharma, Bayer, Janssen, Takeda
Osama Mohamad
Research Funding: Salesforce
Janet E. Cowan
Stock and Other Ownership Interests: GlaxoSmithKline, McKesson
Felix Y. Feng
Stock and Other Ownership Interests: Artera
Consulting or Advisory Role: Janssen Biotech, Astellas Pharma, SerImmune, Foundation Medicine, Exact Sciences, Bristol Myers Squibb, Varian Medical Systems, Novartis, Roivant, Bayer, BlueStar Genomics, Myovant Sciences, Tempus, Artera
Research Funding: Zenith Epigenetics
Peter R. Carroll
Stock and Other Ownership Interests: Nutcracker Therapeutics, Inc
Honoraria: BioPharm Communications, Exact Sciences
Consulting or Advisory Role: Nutcracker Therapeutics, Inc, Insightec, Progenics, Francis Medical, Alessa Therapeutics
Research Funding: Intuitive Surgical (Inst)
No other potential conflicts of interest were reported.
SUPPORT
Supported by UCSF Goldberg-Benioff Program in Translational Cancer Biology (P.R.C., J.E.C., F.Y.F., O.M.)
AUTHOR CONTRIBUTIONS
Conception and design: Derya Tilki, Anthony V. D'Amico
Financial support: Peter R. Carroll
Administrative support: Peter R. Carroll, Anthony V. D'Amico
Provision of study materials or patients: Osama Mohamad, Felix Y. Feng, Peter R. Carroll, Anthony V. D'Amico
Collection and assembly of data: Derya Tilki, Ming-Hui Chen, Hartwig Huland, Markus Graefen, Osama Mohamad, Janet E. Cowan, Peter R. Carroll, Anthony V. D'Amico
Data analysis and interpretation: Derya Tilki, Ming-Hui Chen, Jing Wu, Osama Mohamad, Felix Y. Feng, Peter R. Carroll, Anthony V. D'Amico
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prostate-Specific Antigen Level at the Time of Salvage Therapy After Radical Prostatectomy for PC and the Risk of Death
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Derya Tilki
Honoraria: Janssen, Ipsen, Exact Sciences, Apogepha, AstraZeneca, Advanced Accelerator Applications, Roche, Takeda, miR Scientific
Consulting or Advisory Role: miR Scientific, AstraZeneca, Roche
Research Funding: Janssen
Ming-Hui Chen
Employment: Boehringer Ingelheim
Markus Graefen
Honoraria: Astellas Pharma, Bayer, Takeda, Janssen, Medtronic
Consulting or Advisory Role: Medtronic
Travel, Accommodations, Expenses: Astellas Pharma, Bayer, Janssen, Takeda
Osama Mohamad
Research Funding: Salesforce
Janet E. Cowan
Stock and Other Ownership Interests: GlaxoSmithKline, McKesson
Felix Y. Feng
Stock and Other Ownership Interests: Artera
Consulting or Advisory Role: Janssen Biotech, Astellas Pharma, SerImmune, Foundation Medicine, Exact Sciences, Bristol Myers Squibb, Varian Medical Systems, Novartis, Roivant, Bayer, BlueStar Genomics, Myovant Sciences, Tempus, Artera
Research Funding: Zenith Epigenetics
Peter R. Carroll
Stock and Other Ownership Interests: Nutcracker Therapeutics, Inc
Honoraria: BioPharm Communications, Exact Sciences
Consulting or Advisory Role: Nutcracker Therapeutics, Inc, Insightec, Progenics, Francis Medical, Alessa Therapeutics
Research Funding: Intuitive Surgical (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Morris MJ, Rowe SP, Gorin MA, et al. : Diagnostic performance of 18F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res 27:3674-3682, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hope TA, Goodman JZ, Allen IE, et al. : Metaanalysis of 68Ga-PSMA-11 PET accuracy for the detection of prostate cancer validated by histopathology. J Nucl Med 60:786-793, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Man Kathia, Van Laeken Nick, Schelfhout V, et al. : Piet Ost,18F-PSMA-11 versus 68Ga-PSMA-11 positron emission tomography/computed tomography for staging and biochemical recurrence of prostate cancer: A prospective double-blind randomised cross-over trial. Eur Urol 82:501-509, 2022 [DOI] [PubMed] [Google Scholar]
- 4.Lowrance WT, Breau RH, Chou R, et al. : Advanced prostate cancer: AUA/ASTRO/SUO Guideline PART I. J Urol 205:14-21, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Lowrance WT, Breau RH, Chou R, et al. : Advanced prostate cancer: AUA/ASTRO/SUO Guideline PART II. J Urol 205:22-29, 2021 [DOI] [PubMed] [Google Scholar]
- 6.Tilki D, Chen MH, Wu J, et al. : Adjuvant versus early salvage radiation therapy for men at high risk for recurrence following radical prostatectomy for prostate cancer and the risk of death. J Clin Oncol 3920:2284-2293, 2021 [DOI] [PubMed] [Google Scholar]
- 7.van den Bergh RCN, Cornford P, Gandaglia G, et al. : EAU-EANM-ESTRO-ESUR-SIOG Guidelines on prostate cancer. Part II—2020 Update: Treatment of relapsing and metastatic prostate cancer. Eur Urol 79:263-282, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Agresti A: Categorical Data Analysis (ed 3). Hoboken, NJ, John Wiley & Sons, 2012 [Google Scholar]
- 9.Fisher RA: On the interpretation of Χ2 from contingency tables, and the calculation of P. J R Stat Soc 85:87-94, 1922 [Google Scholar]
- 10.Hollander M, Wolfe D, Chicken E: Nonparametric Statistical Methods (ed 3). Hoboken, NJ, John Wiley & Sons, 2014 [Google Scholar]
- 11.Klein J, Moeschberger M: Survival Analysis: Techniques for Censored and Truncated Data. Norwell, MA, Springer, 2013 [Google Scholar]
- 12.Snapinn SM, Jiang Q, Iglewicz B: Illustrating the impact of a time-varying covariate with an extended Kaplan-Meier estimator. Am Stat 59:301-307, 2005 [Google Scholar]
- 13.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496-509, 1999 [Google Scholar]
- 14.Kaplan El, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 15.Kutner M, Nachtshein C, Neter J: Analysis of factor level means, in, Applied Linear Regression Models (ed 5). New York, NY, McGraw-Hill/Irwin, 2005, pp 756-759 [Google Scholar]
- 16.Cupples LA, Gagnon DR, Ramaswamy R, D’Agostino RB: Age-adjusted survival curves with application in the Framingham Study. Stat Med 14:1731-1744, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Calais J, Armstrong WR, Kishan AU, et al. : Update from PSMA-SRT Trial NCT03582774: A randomized phase 3 imaging trial of prostate-specific membrane antigen positron emission tomography for salvage radiation therapy for prostate cancer recurrence powered for clinical outcome. Eur Urol Focus 7:238-240, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrie C, Magné N, Burban-Provost P, et al. : Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): A 112-month follow-up of a phase 3, randomised trial. Lancet Oncol 20:1740-1749, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Parker CC, Clarke N, Cook A, et al. : Duration of androgen deprivation therapy (ADT) with post-operative radiotherapy (RT) for prostate cancer: First results of the RADICALS-HD trial (ISRCTN40814031). Ann Oncol 33:S808-S869, 2022 [Google Scholar]
- 20.Shipley WU, Seiferheld W, Lukka HR, et al. : Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med 376:417-428, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epstein JI, Allsbrook WC, Jr, Amin MB, et al. : The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol 29:1228-1242, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Parker CC, Clarke NW, Cook AD, et al. : Timing of radiotherapy after radical prostatectomy (RADICALS-RT): A randomised, controlled phase 3 trial. Lancet 396:1413-1421, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]