Keywords: acute kidney injury, 3-deacyl 6-acyl phosphorylated hexaacyl disaccharide, ischemia-reperfusion injury, phosphorylated hexaacyl disaccharide, Toll-like receptor 4 agonist
Abstract
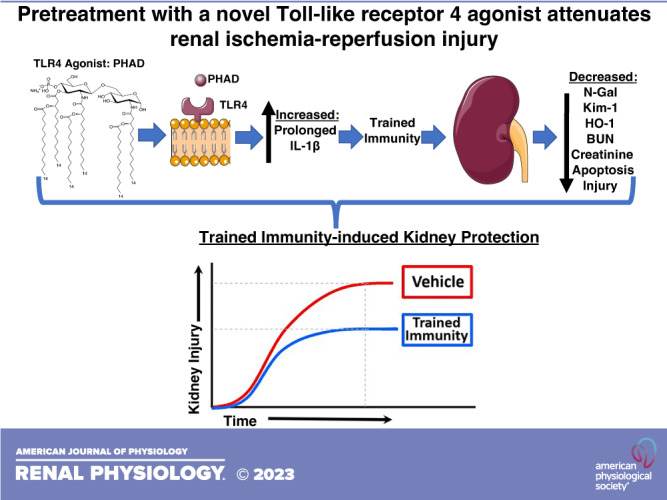
Acute kidney injury (AKI) is common in surgical and critically ill patients. This study examined whether pretreatment with a novel Toll-like receptor 4 agonist attenuated ischemia-reperfusion injury (IRI)-induced AKI (IRI-AKI). We performed a blinded, randomized-controlled study in mice pretreated with 3-deacyl 6-acyl phosphorylated hexaacyl disaccharide (PHAD), a synthetic Toll-like receptor 4 agonist. Two cohorts of male BALB/c mice received intravenous vehicle or PHAD (2, 20, or 200 µg) at 48 and 24 h before unilateral renal pedicle clamping and simultaneous contralateral nephrectomy. A separate cohort of mice received intravenous vehicle or 200 µg PHAD followed by bilateral IRI-AKI. Mice were monitored for evidence of kidney injury for 3 days postreperfusion. Kidney function was assessed by serum blood urea nitrogen and creatinine measurements. Kidney tubular injury was assessed by semiquantitative analysis of tubular morphology on periodic acid-Schiff (PAS)-stained kidney sections and by kidney mRNA quantification of injury [neutrophil gelatinase-associated lipocalin (Ngal), kidney injury molecule-1 (Kim-1), and heme oxygenase-1 (Ho-1)] and inflammation [interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-α (Tnf-α)] using quantitative RT-PCR. Immunohistochemistry was used to quantify proximal tubular cell injury and renal macrophages by quantifying the areas stained with Kim-1 and F4/80 antibodies, respectively, and TUNEL staining to detect the apoptotic nuclei. PHAD pretreatment yielded dose-dependent kidney function preservation after unilateral IRI-AKI. Histological injury, apoptosis, Kim-1 staining, and Ngal mRNA were lower in PHAD-treated mice and IL-1β mRNA was higher in PHAD-treated mice. Similar pretreatment protection was noted with 200 mg PHAD after bilateral IRI-AKI, with significantly reduced Kim-1 immunostaining in the outer medulla of mice treated with PHAD after bilateral IRI-AKI. In conclusion, PHAD pretreatment leads to dose-dependent protection from renal injury after unilateral and bilateral IRI-AKI in mice.
NEW & NOTEWORTHY Pretreatment with 3-deacyl 6-acyl phosphorylated hexaacyl disaccharide; a novel synthetic Toll-like receptor 4 agonist, preserves kidney function during ischemia-reperfusion injury-induced acute kidney injury.
INTRODUCTION
Surgical and critically ill patient populations have a higher predisposition of developing acute kidney injury (AKI). In patients undergoing noncardiac surgery, the incidence of AKI is as high as 12% and is associated with increased mortality and morbidity, including the development of chronic kidney disease (1–4). Patients undergoing cardiac surgery have a higher incidence of AKI ranging from 7% to 40%, with up to 3% developing severe, dialysis-dependent AKI (5). The mechanisms of AKI in these patients are heterogeneous and may involve drug toxicities and heme-induced injury, but perioperative changes in intrarenal blood flow are also thought to contribute to injury by promoting ischemia-reperfusion injury (IRI; 6). Currently, there are no definitive Federal Drug Administration-approved therapies to prevent AKI that occurs after surgery, whereas alternative strategies, such as remote ischemic preconditioning, have shown mixed results as high-quality trials of remote ischemic preconditioning are complex and uncertain (7–11). Thus, there is an unmet need for the prevention of AKI in perioperative medicine. One strategy that shows promise in this field is pretreatment with Toll-like receptor 4 (TLR4) agonists, which have been shown to protect against organ damage associated with cardiac and striated muscle injury, neuronal injury, and sepsis (12–19), but have not been evaluated after IRI-induced AKI (IRI-AKI). Trained immunity is induced upon priming innate leukocytes with pathogen-derived products. This confers innate immune memory, yielding long-term and broad-spectrum resistance to infection (20). The TLR4 ligands, lipopolysaccharide (LPS), or monophosphoryl lipid A (MPLA) induce innate immune memory and lead to a controlled inflammatory cytokine response during the development of a clinically relevant severe infection (17, 21–23). However, LPS is toxic to humans primarily due to the two phosphate groups, and MPLA is no longer available for pharmaceutical development outside of its role as a vaccine adjuvant (24, 25). Three novel, ultrapure synthetic TLR4 agonists, belonging to the phosphorylated hexaacyl disaccharide class, have been shown to induce innate immune broad-spectrum antimicrobial functions for up to 10 days and a regulated cytokine response, but without the toxicity of LPS (26). Unlike MPLA, all three phosphorylated hexaacyl disaccharides are candidates for pharmaceutical development. In this study, we therefore tested the hypothesis that pretreatment with a phosphorylated hexaacyl disaccharide, monophosphoryl hexaacyl lipid A, 3-deacyl 6-acyl phosphorylated hexaacyl disaccharide (PHAD), would confer organ protection in mouse models of IRI-AKI. To our knowledge, this study is the first to characterize PHAD-induced organ protection in models of IRI-AKI.
MATERIALS AND METHODS
Mice
This study was approved by the Institutional Animal Care and Use Committee of Vanderbilt University Medical Center (Protocol No. M1800068-01) and complied with the National Institutes of Health Guide for the Care and Use of Experimental Animals. In accordance with the Guide for the Care and Use of Laboratory Animals and the Public Health Service Policy on Humane Care and Use of Laboratory Animals, mice were housed in an American Association for Accreditation of Laboratory Animal Care-accredited animal facility. Specifically, mice had continuous access to laboratory rodent diet (LabDiet) and water, and lights were maintained on from 6 AM through 9 PM.
Ischemia-Reperfusion Injury-Induced Acute Kidney Injury
Male BALB/c mice (10–11 wk old, 22–25 g) were purchased from Charles River (Hollister, CA) and were allowed to acclimate for 1 wk before experiments. IRI-AKI surgery was performed under ketamine/xylazine anesthesia [120 mg/kg (3 mg) ketamine and 12 mg/kg (0.3 mg) xylazine] administered in 100 μL sterile saline by intraperitoneal injection. Mice underwent two types of surgery to induce IRI-AKI: unilateral IRI-AKI with contralateral nephrectomy (unilateral IRI) and, in separate cohorts, bilateral IRI-AKI. For unilateral IRI, the mouse was placed prone on a heat pad set to 37°C, and incisions were made in the muscle and skin to exteriorize the right kidney for nephrectomy. The adrenal gland was displaced from the kidney, and 5-0 silk suture (LA53G, Ethicon) was tied around the renal artery, vein, and ureter with a double surgical knot before the kidney was removed by cutting distally to the knot. The left kidney was then exteriorized, and fat and connective tissues were dissected from the renal pedicle to expose the renal artery and vein. A vascular clamp (Cat. No. RS-5459, Roboz) was applied to the renal vasculature for 28 min. The kidney was tucked back under the skin, and the mouse was kept warm on the heat pad during the 28 min of ischemia time. For bilateral IRI-AKI, mice underwent bilateral renal artery ischemia for 24 min without nephrectomy. Successful ischemia was confirmed by a dark purple appearance of the kidney before clamp removal, and successful reperfusion was confirmed by a return to the normal pink color of the kidney within 1 or 2 min of clamp removal. Upon removal of the vascular clamp, the muscle wall was closed with 6-0 absorbable suture (J492G, Ethicon) followed by skin closure with 7-mm wound clips (RS-9255 and RS-9250, Roboz). Upon skin closure, mice received 0.5 mL warm sterile saline subcutaneously immediately after surgery and at 24 h after surgery. Analgesia was provided with buprenorphine subcutaneously immediately after surgery and every 12 h for 3 days. Sham-operated mice underwent the same surgical approaches (for unilateral IRI-AKI, they underwent right nephrectomy, but the left renal pedicle was not clamped; for bilateral IRI-AKI, both kidneys were exteriorized but the renal pedicles were not clamped). Blood was collected at baseline (before injury) and on days 1 and 3 after injury by submandibular vein puncture into lithium heparin-coated microcuvette tubes, and plasma was frozen at −80°C for blood urea nitrogen (BUN) and creatinine assays. On day 3 post-IRI, mice underwent terminal anesthesia using 5% isoflurane; upon achievement of a respiratory rate of 5 breaths/min, laparotomy and sternotomy were performed, and terminal day 3 blood samples were collected by direct left ventricular apical cardiac puncture followed by cervical dislocation. BUN levels were measured by adding 200 μL Infinity Urea reagent (Cat. No. TR12421, Thermo Scientific) to 2 μL plasma or urea standard (B7550-STD, Pointe Scientific) in duplicate in a 96-well plate (Cat. No. 130188, Thermo Scientific). Absorbance was measured at 340 and 405 nm, and urea nitrogen was calculated as described by the manufacturer’s instructions. Serum creatinine was assessed by liquid chromatography-mass spectrometry (LC-MS) at the O’Brien Core Center for Acute Kidney Injury Research at the University of Alabama at Birmingham.
Treatment With PHAD
PHAD was synthesized de novo by Avanti Polar Lipids (Lot 699852 P-1MG-B-012, Alabaster, AL), dissolved in endotoxin-free water containing 0.2% trimethylamine, and sonicated in a 40°C-water bath for 1 h. Dilutions of PHAD were prepared for intravenous injection using lactated Ringer’s solution. Intravenous priming was performed via a penile vein injection using vehicle or PHAD at 2, 20, or 200 μg/0.2 mL per mouse. Vehicle or PHAD was administered at 48 and 24 h before IRI-AKI.
Randomization and Blinding
Mice were randomized into the following five groups: sham, vehicle treatment, and treatment with intravenous 2, 20, or 200 μg PHAD per mouse (n = 10). Randomization was accomplished using a website random team generator (Random Lists, https://www.randomlists.com/team-generator). Cages were not randomized, but rather individual mice were randomized upon opening each cage, according to the randomization table generated by the website. The randomizer assigns mice from each group to each cage. The first mouse of cage 1 was selected at random and marked according to the first-assigned mouse on the randomization table and the second mouse was selected at random and marked according to the second-assigned mouse on the randomization table, and so on. This allowed us to control for any differences that may exist between cages, although these cages were located immediately adjacent to each other to control for light cycle and animal care handlers. Scientists injecting the mice, performing the surgeries, and conducting the data analyses were blinded as to the treatment groups until each of the analyses had been completed (including BUN, quantitative RT-PCR, histology, and immunostaining experiments).
Histological Analysis of Tubular Injury
Upon euthanasia, the right atrium was cannulated with a 22-gauge needle, and perfusion was performed with 10 mL of 1X PBS. The right kidney pedicle was ligated and the kidney was excised. The 22-gauge needle was kept in situ, and the left kidney was perfused using 10 mL of 10% formalin fixation solution. The left kidney was excised and cut along the transverse plane, and two 2-mm sections were excised from the center of each kidney half; one section was minced and stored at −80°C and the other section was immediately immersed in 10% formalin for 4 h. Upon completion of the 4-h 10% formalin fixation, the tissue was washed three times in PBS and then submersed in 70% ethanol overnight at 4°C. The following day, kidney sections were processed and embedded in paraffin using standard histological techniques. Five-micrometer sections were deparaffinized and hydrated by standard methods and then stained with period acid-Schiff (PAS) using a PAS staining kit (Cat. No. 395B-1KT, Sigma) as described by the manufacturer. Semiquantitative histological scoring of the outer stripe of the outer medulla (OSOM) of each section was performed on a five-point severity scale of tubular degeneration/necrosis where 0 = within normal limits, 1 = minimal, 2 = mild, 3 = moderate, 4 = marked, and 5 = severe. An aggregate score for the whole region of interest was given for each section by a pathologist blinded to the treatment group (L. Himmel).
Immunohistochemistry Staining and Quantification
Animals in the control group served as the internal baseline for the purpose of this study. Immunofluorescence controls were performed during optimization of the immunofluorescence antibodies and served as non-vehicle and non-PHAD controls. Formalin-fixed, paraffin-embedded kidney sections were deparaffinized and rehydrated in preparation for immunofluorescence labeling. Slides were heated in a steamer for 26 min in Target Retrieval Solution [citrate (pH 6.1), Cat. No. S169984-2, Agilent). They were washed and then blocked with an Avidin/Biotin Blocking kit (H-4000, Vector Labs) and Power Block (Cat. No. HK085, BioGenex) diluted in PBS for 30 min before incubation with primary antibodies. Each section was incubated overnight at 4°C with Lotus tetragonolobus lectin (LTL)-biotin (B-1325, Vector Labs), rat anti-F4/80 monoclonal antibody diluted to 1/250 (CI:A3-1; ab6640, Abcam), or rat anti-kidney injury molecule-1 (Kim-1) monoclonal antibody (Cat. No. MAB1817, R&D Systems) diluted to 1/500 in 2.5% BSA and 0.05% Tween 20 in PBS, as previously described (27, 28). The following day, slides were washed and incubated with NeutrAvidin protein, DyLight 488 (Cat. No. 84607, Thermo Fisher), and Cy5 AffiniPure donkey anti-rat IgG (H + L; Cat. No. 712-175-153, Jackson ImmunoResearch) diluted to 1/500 in 2.5% BSA and 0.05% Tween 20 in PBS. Sections were thoroughly washed and incubated overnight with directly conjugated antibody. Nuclei were labeled using Hoechst 33342 (Cat. No. 62249, Thermo Fisher) diluted to 1:5,000 in PBS. Slides were mounted in ProLong Gold Antifade Mountant (P10144, Thermo Fisher) and air-dried overnight. Digital images were acquired by scanning the stained slides using a ZEISS AxioScan.Z1. Colabeling of LTL with the anti-Kim-1 monoclonal antibody under these conditions shows correct localization of Kim-1 limited to LTL-positive proximal tubules after IRI-AKI but lack of staining in uninjured kidneys, whereas the F4/80 monoclonal antibody shows correct F4/80 immunostaining limited to the interstitial space surrounding tubules after IRI-AKI, with only sparse interstitial staining in uninjured kidneys (Supplemental Fig. S1).
Quantitative analysis of digital images was completed under blinded conditions using QuPath v0.3.0 (29). Regions of interest were manually annotated using LTL staining and the arcuate vessels as landmarks, enabling signals in the cortex and OSOM to be quantified separately. Areas of immunopositivity per region per kidney were recorded for Kim-1 and F4/80, and results are presented as means ± SE and individual data points are shown.
TUNEL Staining and Analysis
Formalin-fixed, paraffin-embedded kidney sections were deparaffinized and rehydrated in preparation for TUNEL staining. Slides were placed on the Leica Bond RX IHC Stainer. All steps besides dehydration, clearing, and coverslipping are performed on the Bond RX. Slides were deparaffinized. Antigen retrieval was performed on the Bond RX using Triton X-100 (Cat. No. T9284) for 5 min. Slides were then incubated with Equilibration Buffer (G7130, Promega, Madison, WI) for 5 min followed by the terminal deoxynucleotidyl transferase reaction mix (G7130, Promega) for 10 min and saline sodium citrate-20x (G7130, Promega) for 10 min. Slides were incubated with streptavidin-horseradish peroxidase (RE7104, Novocastra, Newcastle Upon Tyne, UK) for 5 min and the Bond Refine detection system (Cat. No. DS9800, Leica, Buffalo Grove, IL) was used for visualization. After staining, sections were counterstained using hematoxylin (Cat. No. 7211, Epredia) as outlined in the manufacturer’s instruction, dehydrated, cleared, and coverslipped. Digital images were acquired by scanning the stained slides using a ZEISS AxioScan.Z1, and quantitative analysis of digital images was completed under blinded conditions using QuPath v0.3.0 (29). The OSOM was manually annotated using the arcuate vessels as outer landmarks and S3 segment proximal tubules as inner landmarks. All tubules were selected and classified as having viable or containing necrotic cellular casts. Once necrotic cast-filled tubules were excluded, a positive cell detector was run within QuPath to detect all nuclei (counterstained using hematoxylin) and diaminobenzidine (TUNEL)-positive nuclei using the same threshold for all of the stained sections. The output data identified all positively stained nuclei in the defined areas and results are expressed as the percentage of TUNEL positive/total nuclei in the OSOM. Masked areas containing DAB-positive necrotic casts were excluded.
Quantitative Real-Time PCR
A central transverse kidney section was lysed in a Lysing Matrix A tube (MP Biomedicals, Irvine, CA) using a MiniBeater-16 (BioSpec Products, Bartlesville, OK), and RNA was isolated using a RNeasy Mini kit (Qiagen, Hilden, Germany). cDNA was synthesized from 1 μg RNA using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Quantitative mRNA expression was determined by real-time PCR using iQ SYBR Green Supermix (Bio-Rad) with the indicated pairs of primers (Table 1).
Table 1.
Primer pairs for kidney tissue mRNA
| Primer | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| Gapdh | TGGAGAAACCTGCCAAGTATGA | GAAGAGTGGGAGTTGCTGTTGA |
| N-Gal | GCAGGTGGTACGTTGTGGG | CTCTTGTAGCTCATAGATGGTGC |
| Kim-1 | ACAAACCAGACTGGAATGGC | GTCCACAAGGAGCAGTAGCA |
| IL-6 | CTTCCATCCAGTTGCCTTCTTG | AATTAAGCCTCCGACTTGTGAAG |
| Tnf-α | CGGAGTCCGGGCAGGT | CGGAGTCCGGGCAGGT |
| IL-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| Irf-1 | ATGCCAATCACTCGAATGCG | TTGTATCGGCCTGTGTGAATG |
| Ccl-2 | TGCATCTGCCCTAAGGTCTTC | AAGTGCTTGAGGTGGTTGTGG |
| Ccl-3 | TGCCCTTGCTGTTCTTCTCT | GATGAATTGGCGTGGAATCT |
| Ho-1 | ACAGAGGAACACAAAGACCAG | GTGTCTGGGATGAGCTAGTG |
Real-time PCR was performed in duplicate on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad) and quantification of gene expression was determined by the ΔΔ cycle threshold (ΔCt) method, normalized for Gapdh mRNA expression, as previously described (30).
Statistics
All data were analyzed using GraphPad Prism software version 9.3.1 (GraphPad Software, San Diego, CA). Between-group analyses were performed using a Mann–Whitney U test to compare two groups and from multiple group experiments by nonparametric one-way ANOVA (Kruskal Wallis test) for between-group testing using Dunnett’s post hoc correction for multiple between-group comparisons. We also used two-way ANOVA to compare groups followed over time followed by Sidak’s post hoc correction for multiple between-group comparisons, and survival curves were compared by a log-rank test. Values of P < 0.05 were considered statistically significant.
RESULTS
PHAD Pretreatment Preserves Renal Function and Reduces Renal Injury After Unilateral IRI-AKI
There was a clear, dose-dependent decrease in IRI-induced kidney injury, as determined by reduced elevations in BUN and creatinine levels, in mice pretreated with intravenous PHAD (Fig. 1A). Intravenous PHAD treatment also reduced tubular injury in the cortex and OSOM, with reduced interstitial edema, and there were fewer casts with increasing doses of PHAD (Fig. 1, B and C). There was no significant improvement in survival in PHAD-treated mice, but more mice survived at the 2- and 20-µg pretreatment doses compared with the vehicle control group (Fig. 1D). In addition, there was a reduction in expression of the distal tubular injury marker Ngal but no reduction in proximal tubular injury marker Kim-1 mRNA in mice treated with 200 µg PHAD (Fig. 1E). This was associated with a dose-dependent increase in IL-1β but not Tnf-α or IL-6 mRNAs compared with vehicle control mice (Fig. 1E).
Figure 1.
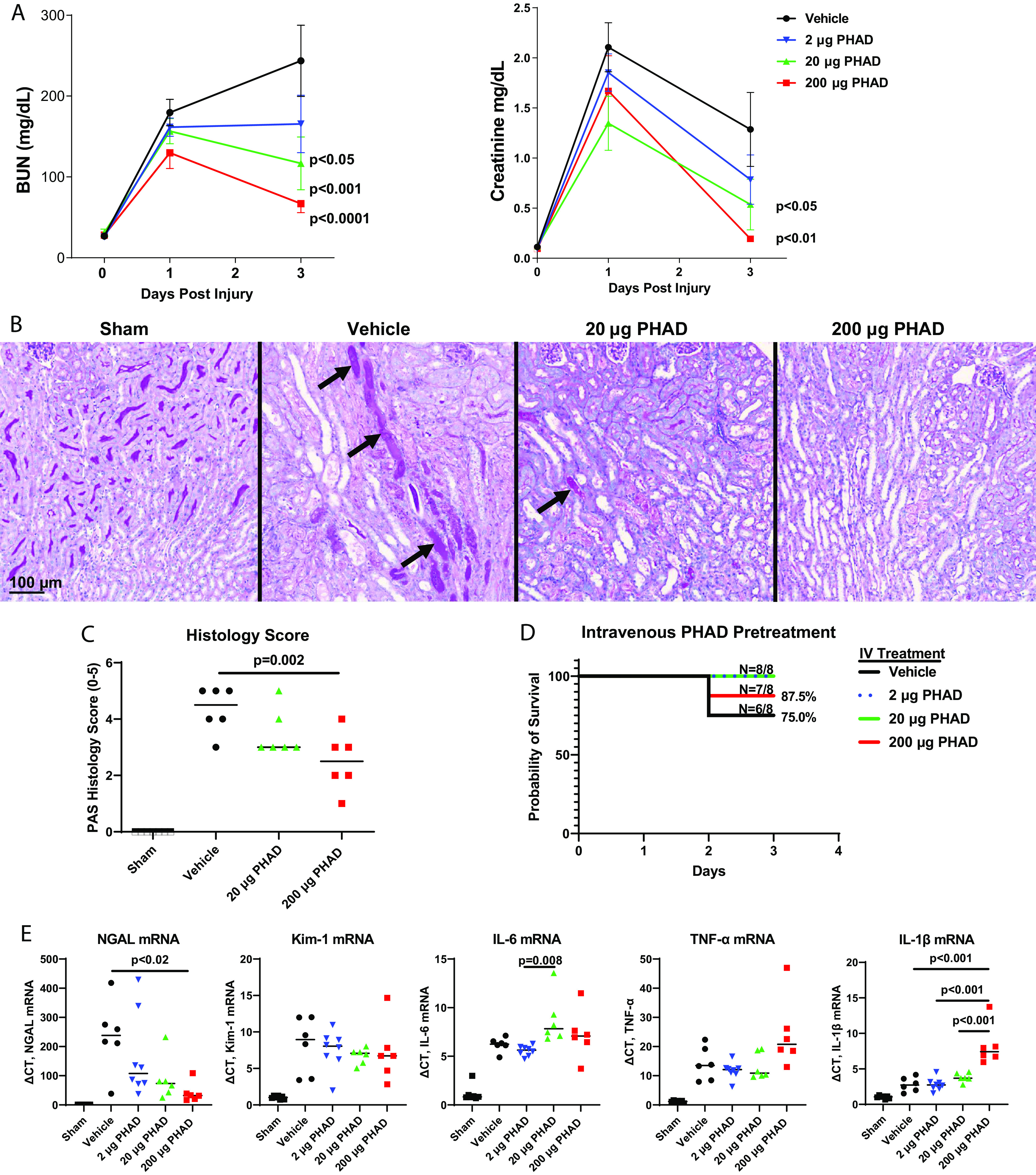
Pretreatment with 3-deacyl 6-acyl phosphorylated hexaacyl disaccharide (PHAD) in mice that underwent unilateral ischemia-reperfusion injury-induced acute kidney injury. Mice were pretreated with intravenous (IV) PHAD at 2, 20, and 200 µg/mouse or vehicle control 48 and 24 h prior to undergoing right nephrectomy followed by clamping of the left renal pedicle for 28 min. A: blood was analyzed for blood urea nitrogen (BUN) and creatinine at baseline (day 0) and postinjury days 1 and 3. Results are expressed as means ± SE with n = 8. Two-way ANOVA was used to compare differences between PHAD- and vehicle-treated mice over time, with P values indicated. B: representative images of periodic acid-Schiff (PAS)-stained sections of the outer medulla day 3 after injury in sham, vehicle-treated, and PHAD-treated mice. Arrows point to casts within the collecting tubules. Scale bar = 100 µm. C: median tubular injury scores in the outer stripe of the outer medulla from PAS-stained sections day 3 after injury in sham, vehicle-treated, and PHAD-treated mice. D: 3-day survival curves. Group differences were compared by a log-rank test (P > 0.05). E: median quantitative RT-PCR for renal expression of neutrophil gelatinase-associated lipocalin (Ngal), kidney injury molecule-1 (Kim-1), interleukin-6 (IL-6), tumor necrosis factor-α (Tnf-α), and interleukin-1β (IL-1β) mRNAs. GAPDH was used as the internal control for quantitative RT-PCR, and change in threshold cycle (ΔCt) values is graphed relative to the sham group. For C and D, individual data points and medians are shown and between-group differences were compared by one-way ANOVA using Dunnett’s post hoc correction for multiple between-group comparison Kruskal–Wallis test, with significant P values (<0.05) indicated. n = 6.
PHAD Preserves Renal Function and Reduces Renal Injury After Bilateral IRI-AKI
We also evaluated the effects of pretreatment with PHAD at 200 µg/mouse on renal function and tissue injury after bilateral IRI-AKI. Bilateral IRI-AKI has different pathophysiology compared with unilateral IRI-AKI since removal of the contralateral kidney in unilateral IRI-AKI increases blood flow to the remaining injured kidney, accelerating recovery compared with bilateral IRI-AKI (6, 31). Renal injury was markedly reduced, as assessed by reduced BUN and creatinine levels, at 24 and 72 h after bilateral IRI-AKI in intravenous PHAD-treated mice (Fig. 2A), but there was no difference in survival between groups (Supplemental Fig. S2). PHAD treatment also reduced tubular injury, with less interstitial edema and fewer tubular casts (Fig. 2, B and D), and apoptosis (Fig. 2, C and E). As with unilateral IRI-AKI, there were lower levels of Ngal mRNA and increased IL-1β mRNA in PHAD-treated mouse kidneys compared with vehicle-treated mouse kidneys, and there were no differences in renal Kim-1, IL-6, or Tnf-α mRNAs (Fig. 2F). There was, however, a significant reduction in the area of cells expressing Kim-1 protein in the outer medulla but not in the cortex of mice treated with PHAD, indicating that there is a dissociation between Kim-1 mRNA and protein detection in these experiments, but there was no difference in F4/80 staining for renal macrophages (Fig. 3). Consistent with these findings, renal expression of Ho-1 mRNA, a ubiquitous injury response marker that is induced in the kidney after injury (32), was also reduced in PHAD-treated mice (Fig. 4A). We also examined interferon regulatory factor (Irf)2, chemokine (C-C motif) ligand (Ccl)2, and Ccl3, given their roles in mediating inflammation-induced macrophage recruitment and polarization, as well as polymorphonuclear leukocyte recruitment, but we saw no differences in expression of inflammatory markers Irf1, Ccl2, or Ccl3 mRNAs (Fig. 4, B–D).
Figure 2.
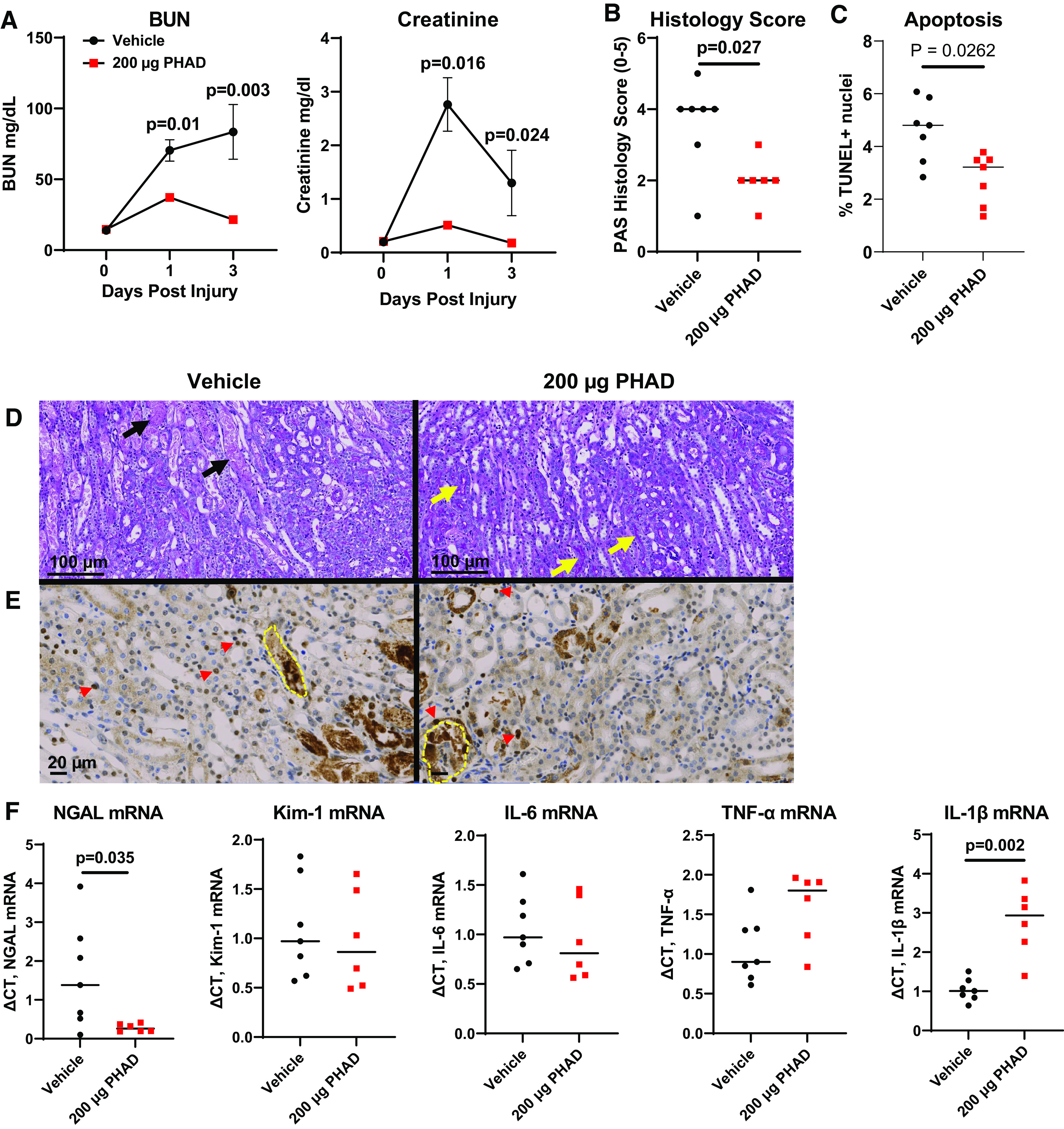
Pretreatment with 3-deacyl 6-acyl phosphorylated hexaacyl disaccharide (PHAD) in mice that underwent bilateral ischemia-reperfusion injury-induced acute kidney injury. Mice were pretreated with intravenous PHAD at 200 µg/mouse or vehicle control 48 and 24 h prior to undergoing bilateral renal pedicle clamping for 24 min. A: blood was analyzed for blood urea nitrogen (BUN) and creatinine at baseline (day 0) and postinjury days 1 and 3. Results are expressed as means ± SE with n = 10. Two-way ANOVA was used to evaluate between-group differences over time (P < 0.05 for both BUN and serum creatinine), with P values shown after Sidak’s correction for multiple post hoc between-group comparisons at each time point. B: tubular injury scores in the outer stripe of the outer medulla from periodic acid-Schiff (PAS)-stained sections day 3 after injury. C: apoptosis in the outer stripe of the outer medulla from TUNEL-stained sections day 3 after injury. D: representative images of PAS-stained sections of the outer medulla day 3 after injury in sham, vehicle-treated, and PHAD-treated mice. Black arrows point to severely injured tubules showing flattening of the surviving epithelium and the presence of cellular debris within the tubular lumen. Yellow arrows indicate PAS-positive brush borders of uninjured S3 segment proximal tubule epithelial cells. Scale bar = 100 µm. E: representative images of TUNEL-stained sections of the outer medulla day 3 after injury in vehicle- and PHAD-treated mice. Red arrowheads indicate examples of TUNEL-positive tubular nuclei. Yellow dotted lines indicate necrotic TUNEL-positive tubular casts excluded from the analysis. Scale bar = 20 µm. F: quantitative RT-PCR for renal expression of neutrophil gelatinase-associated lipocalin (Ngal), kidney injury molecule-1 (Kim-1), interleukin-6 (IL-6), tumor necrosis factor-α (Tnf-α), and interleukin-1β (IL-1β) mRNAs day 3 after injury. GAPDH was used as the internal control for quantitative RT-PCR, and change in threshold cycle (ΔCt) values is graphed relative to the vehicle group. For B, C, and F, individual data points and medians are shown, and P values with a significant difference are shown from a Mann–Whitney U test used to compare groups. n = 6 or 7.
Figure 3.

Immunofluorescence staining for kidney injury molecule-1 (Kim-1) and F4/80 after bilateral ischemia-reperfusion injury-induced acute kidney injury in mice pretreated with 3-deacyl 6-acyl phosphorylated hexaacyl disaccharide (PHAD). Mice were pretreated with intravenous PHAD at 200 µg/mouse or vehicle control 48 and 24 h prior to undergoing bilateral renal pedicle clamping for 24 min. A: representative images stained for expression of Kim-1, expressed by injured proximal tubules (left), and F4/80, expressed by renal macrophages (right), in sequential sections colabeled with Lotus tetragonolobus lectin (LTL) to mark proximal tubular cells in vehicle-treated vs. PHAD-treated mice 3 days after injury. White dotted lines indicate the junction between the cortex and outer medulla, which is only seen in top images. Scale bar = 50 µm. B: quantification of Kim-1- and F4/80-positive areas in the cortex (right) and outer stripe of the outer medulla (left) in vehicle- and PHAD-treated mice at day 3 after injury. Individual data points and medians are shown, and P values are shown from a Mann–Whitney U test used to compare groups. n = 8.
Figure 4.
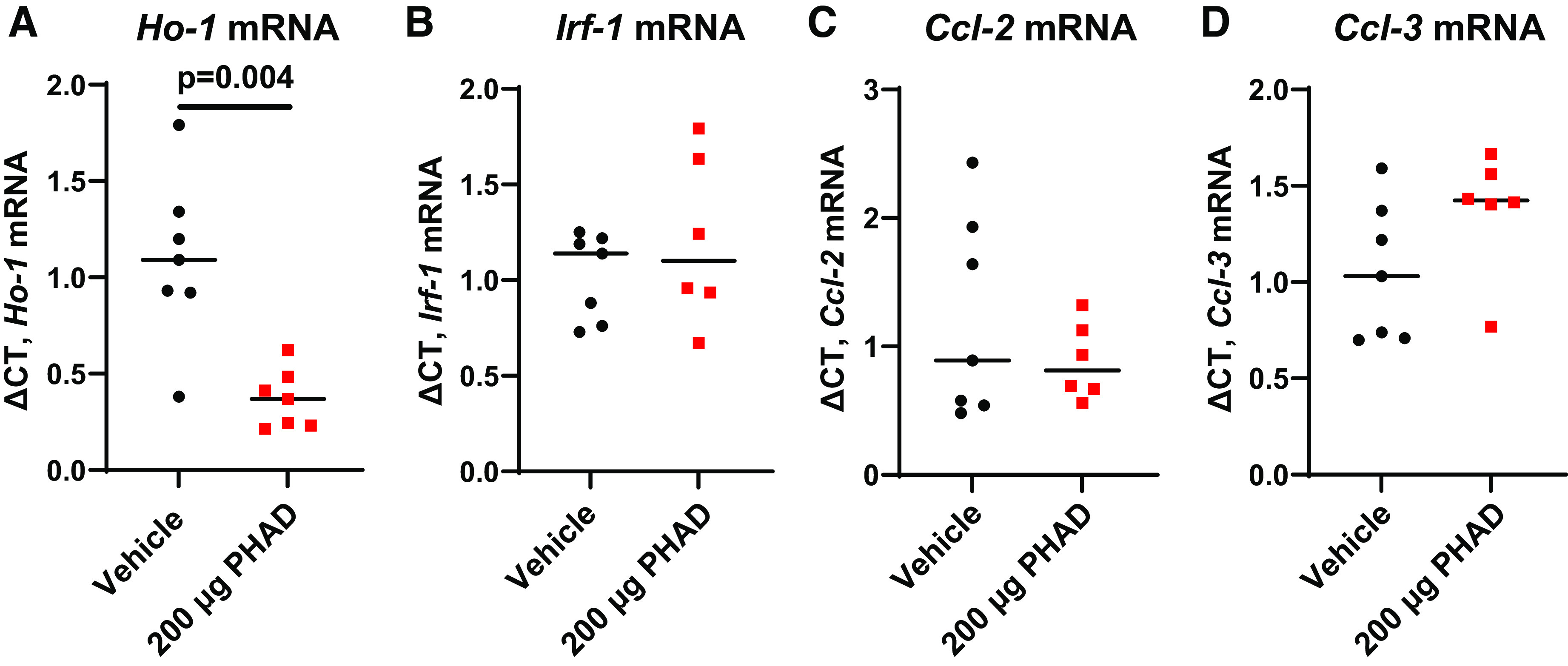
Pretreatment with intravenous 3-deacyl 6-acyl phosphorylated hexaacyl disaccharide (PHAD) in mice that underwent bilateral ischemia-reperfusion injury-induced acute kidney injury. Mice were pretreated with intravenous PHAD at 200 µg/mouse or vehicle control 48 and 24 h prior to undergoing bilateral renal pedicle clamping for 24 min. Quantitative RT-PCR for renal expression of heme oxygenase-1 (Ho-1; A), interferon regulatory factor-1 (Irf1; B), chemokine (C-C motif) ligand 2 (Ccl2; C), and chemokine (C-C motif) ligand 3 (Ccl3; D) day 3 after injury. GAPDH was used as the internal control for quantitative RT-PCR, and change in threshold cycle (ΔCt) values is graphed relative to the vehicle-treated group. Individual data points and medians are shown, and P values are shown from a Mann-Whitney U test used to compare groups. n = 6 or 7.
DISCUSSION
This study demonstrates, for the first time, that pretreatment with the TLR4 agonist PHAD improves renal function and reduces renal tubular injury in two different mouse models of IRI-AKI. The two IRI-AKI models have different pathophysiologies as removal of the contralateral kidney in unilateral IRI-AKI increases blood flow to the remaining injured kidney, accelerating recovery compared with bilateral IRI-AKI (6, 31). Pretreatment with intravenous PHAD provided clear, dose-dependent organ protection from unilateral IRI-AKI. Upon identifying the optimal dose of PHAD for conferring protection against unilateral IRI-AKI, we tested our hypothesis in a separate cohort of mice after bilateral IRI-AKI and observed organ protection. These results demonstrate that PHAD pretreatment provides equipotent organ protection in two models of perioperative AKI. While the translational significance of these two different models for human AKI are unclear, by showing a beneficial effect of PHAD pretreatment in two models of IRI-AKI with different pathophysiologies, these findings indicate that the beneficial effects of PHAD are more robust than if only effective in one of the models.
In our study, PHAD was noted to reduce expression of the distal tubular injury marker Ngal after IRI-AKI. We found no statistically significant differences in Kim-1 mRNA expression but did observe a reduction in the surface area of cells staining with Kim-1 in PHAD-treated mice after bilateral IRI-AKI. The decrease in Kim-1 staining was paralleled by the reduction in histological evidence of proximal tubular injury and apoptosis as well as reduced expression of Ho-1 mRNA, a ubiquitous injury response marker that is induced in the kidney after injury (32). This suggests that the reduction in Kim-1 protein detection reflects reduced proximal tubular cell injury in PHAD-treated mice after IRI-AKI. These findings were unexpected since, in general, similar increases in Kim-1 mRNA and protein levels are usually detected in kidneys after IRI-AKI (33, 34). However, this is not the case with all types of AKI. For example, in a study of patients with sepsis-associated AKI, KIM-1 mRNA was increased in all patients with AKI, whereas KIM-1 protein levels were not (35). The antibody used to detect KIM-1 protein in these studies was raised against a recombinant protein comprising the entire extracellular domain of KIM-1 (mouse anti-human KIM-1, clone 219211, R&D Systems, KIM-1 residues Ser21–Thr288). Since the extracellular domain of KIM-1 undergoes proteolytic cleavage by metalloproteases (36, 37), it is possible that this reduction in KIM-protein detection occurs because KIM-1 is undergoing cleavage of the extracellular domain and is therefore no longer detectable using this antibody. In our study, we used an antibody that was also raised against the extracellular domain of Kim-1 protein (rat anti-mouse Kim-1, clone 222414, R&D Systems, Kim-1 residues Tyr22–Thr212), so the reduction in Kim-1 protein detection may also have resulted from increased Kim-1 cleavage in PHAD-treated mice after IRI-AKI. Interestingly, metalloprotease-mediated cleavage of Kim-1 is increased by exposure to inflammatory cytokines including TNF-α (38). This suggests that reduced detection of Kim-1 protein versus Kim-1 mRNA levels in sepsis-associated AKI, and in PHAD-treated mice after IRI-AKI, may also have resulted from increased Kim-1 protein cleavage in the inflammatory milieu of these kidneys.
Pretreatment with the TLR4 agonist MPLA has demonstrated protection against myocardial IRI in rabbit (12), canine (13), and swine (14) models, as well as reduction in latissimus dorsi muscle flap necrosis in a rat model of muscle flap surgery (16). In our laboratory, we observed that pretreatment with intravenous MPLA followed by a challenge of Staphylococcus aureus (1 × 108 colony-forming units) was associated with an increase in kidney macrophages by immunohistochemistry, where MPLA-pretreated mice showed increased infiltration of macrophages, distributed within the interstitium between renal tubules (17). Our previous study has shown that pretreatment with TLR4 agonists reprograms macrophage function to facilitate the development of trained immunity, which protects against infection and inflammation-induced injury (17). IL-1β has been shown to directly induce trained immunity in human monocytes via epigenetic reprogramming mechanisms (39), and it also enhances glycolysis in hematopoietic progenitor cells (40). Consistent with these findings, we observed a significant dose-dependent increase in expression of IL-1β mRNA. In contrast, prior studies have shown that pretreatment with LPS (a classical TLR4 agonist) before renal IRI causes a sustained increase in renal Tnf-α or IL-6 mRNA in PHAD-treated mice. These findings suggest that treatment with PHAD induces a controlled cytokine response, which is regulated and sustained upon the ischemic insult and is associated with renal protection after IRI. Although we did not observe any changes in renal macrophage numbers by F4/80 staining in PHAD-treated mice, we anticipate that macrophage function will be altered in PHAD-treated mice.
Our study has limitations. We selected male mice to avoid confounders from the estrous cycle. However, female mice are more resistant to the effects of IRI-AKI (30) and may show different responses to PHAD pretreatment than male mice. We also used only healthy mice without the typical comorbidities that are associated with postoperative AKI in patients (such as diabetes and old age). Although we anticipate similar results in female and older mice, our findings are specific to 10- to 11-wk-old male mice only.
Perspectives and Significance
Our findings indicate that intravenous administration of PHAD before injury provides marked, dose-dependent protective effects in two mouse models of IRI-AKI. Further studies will be required to elucidate the potential role of trained immunity in PHAD-induced protection against renal IRI. Nevertheless, our findings indicate that intravenous PHAD is protective in experimental IRI-AKI.
DATA AVAILABILITY
No unique resources or reagents have been generated with this work. Original quantitative data will be provided by the authors on request.
SUPPLEMENTAL DATA
Supplemental Figs. S1 and S2: https://doi.org/10.6084/m9.figshare.22191601.v1.
GRANTS
This work was supported by National Institutes of Health Grants K08GM123345 (to A.H.), 5T32GM108554-05 (to N.K.P.), R35GM141927 (to J.K.B.), R01AI151210 (to E.R.S.), R01GM119197 (to E.R.S.), UC2DK126122 (to M.P.d.C.), and R01DK112688 (to M.P.d.C.) and by Department of Defense Grant W81XWH-17-1-0610 (to M.P.d.C.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
M.D.C. provides consultancy advice for Johnson & Johnson.
AUTHOR CONTRIBUTIONS
A.H. conceived and designed research; A.H., N.K.P., M.B., R.D., L.N.L., J.K.B., and A.M.O. performed experiments; A.H., N.K.P., M.B., L.H., and M.P.d.C. analyzed data; A.H. and M.P.d.C. interpreted results of experiments; A.H., L.H., and M.P.d.C. prepared figures; A.H. drafted manuscript; A.H., N.K.P., M.B., R.D., L.H., L.N.L., J.K.B., E.R.S., and M.P.d.C. edited and revised manuscript; A.H. and M.P.d.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the O’Brien Core Center for Acute Kidney Injury Research at the University of Alabama at Birmingham for analyzing our mouse plasma creatinine and the Vanderbilt Translational Pathology Shared Resource Core for the assistance in preparing the histological specimens for TUNEL stain analysis.
REFERENCES
- 1. Grams ME, Sang Y, Coresh J, Ballew S, Matsushita K, Molnar MZ, Szabo Z, Kalantar-Zadeh K, Kovesdy CP. Acute kidney injury after major surgery: a retrospective analysis of Veterans Health Administration data. Am J Kidney Dis 67: 872–880, 2016. doi: 10.1053/j.ajkd.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cho E, Kim SC, Kim MG, Jo SK, Cho WY, Kim HK. The incidence and risk factors of acute kidney injury after hepatobiliary surgery: a prospective observational study. BMC Nephrol 15: 169, 2014. doi: 10.1186/1471-2369-15-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saran R, Robinson B, Abbott KC, Agodoa LYC, Bhave N, Bragg-Gresham J, , et al. US Renal Data System 2017 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis 71: A7, 2018. [Erratum in Am J Kidney Dis 2018;71: 501]. doi: 10.1053/j.ajkd.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biteker M, Dayan A, Tekkeşin Aİ, Can MM, Taycı İ, İlhan E, Şahin G. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg 207: 53–59, 2014. doi: 10.1016/j.amjsurg.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 5. Callejas R, Panadero A, Vives M, Duque P, Echarri G, Monedero P; Renal Dysfunction in Cardiac Surgery Spanish Group (GEDRCC2). Preoperative predictive model for acute kidney injury after elective cardiac surgery: a prospective multicenter cohort study. Minerva Anestesiol 85: 34–44, 2019. doi: 10.23736/S0375-9393.18.12257-7. [DOI] [PubMed] [Google Scholar]
- 6. Hukriede NA, Soranno DE, Sander V, Perreau T, Starr MC, Yuen PST, Siskind LJ, Hutchens MP, Davidson AJ, Burmeister DM, Faubel S, de Caestecker MP. Experimental models of acute kidney injury for translational research. Nat Rev Nephrol 18: 277–293, 2022. doi: 10.1038/s41581-022-00539-2. [DOI] [PubMed] [Google Scholar]
- 7. Lei VJ, Luong T, Shan E, Chen X, Neuman MD, Eneanya ND, Polsky DE, Volpp KG, Fleisher LA, Holmes JH, Navathe AS. Risk stratification for postoperative acute kidney injury in major noncardiac surgery using preoperative and intraoperative data. JAMA Netw Open 2: e1916921, 2019. doi: 10.1001/jamanetworkopen.2019.16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, Wolf B, Goebel U, Schwer CI, Rosenberger P, Haeberle H, Görlich D, Kellum JA, Meersch M; for the RenalRIPC Investigators. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 313: 2133–2141, 2015. doi: 10.1001/jama.2015.4189. [DOI] [PubMed] [Google Scholar]
- 9. Zarbock A, Kellum JA. Remote ischemic preconditioning and protection of the kidney–a novel therapeutic option. Crit Care Med 44: 607–616, 2016. doi: 10.1097/CCM.0000000000001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Long YQ, Feng XM, Shan XS, Chen QC, Xia Z, Ji FH, Liu H, Peng K. remote ischemic preconditioning reduces acute kidney injury after cardiac surgery: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg 134: 592–605, 2022. doi: 10.1213/ANE.0000000000005804. [DOI] [PubMed] [Google Scholar]
- 11. Murphy N, Vijayan A, Frohlich S, O'Farrell F, Barry M, Sheehan S, Boylan J, Conlon N. Remote ischemic preconditioning does not affect the incidence of acute kidney injury after elective abdominal aortic aneurysm repair. J Cardiothorac Vasc Anesth 28: 1285–1292, 2014. doi: 10.1053/j.jvca.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 12. Elliott GT, Comerford ML, Smith JR, Zhao L. Myocardial ischemia/reperfusion protection using monophosphoryl lipid A is abrogated by the ATP-sensitive potassium channel blocker, glibenclamide. Cardiovasc Res 32: 1071–1080, 1996. doi: 10.1016/s0008-6363(96)00154-x. [DOI] [PubMed] [Google Scholar]
- 13. Elliott GT, Mei DA, Gross GJ. Monophosphoryl lipid A attenuates myocardial stunning in dogs: role of ATP-sensitive potassium channels. J Cardiovasc Pharmacol 32: 49–56, 1998. doi: 10.1097/00005344-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 14. Yoshida T, Engelman RM, Engelman DT, Rousou JA, Maulik N, Sato M, Elliott GT, Das DK. Preconditioning of swine heart with monophosphoryl lipid A improves myocardial preservation. Ann Thorac Surg 70: 895–900, 2000. doi: 10.1016/s0003-4975(00)01508-3. [DOI] [PubMed] [Google Scholar]
- 15. Venezia S, Refolo V, Polissidis A, Stefanis L, Wenning GK, Stefanova N. Toll-like receptor 4 stimulation with monophosphoryl lipid A ameliorates motor deficits and nigral neurodegeneration triggered by extraneuronal α-synucleinopathy. Mol Neurodegener 12: 52, 2017. doi: 10.1186/s13024-017-0195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maldonado C, Stadelmann WK, Ramirez S, Quan EE, Barker JH. Preconditioning of latissimus dorsi muscle flaps with monophosphoryl lipid a. Plast Reconstr Surg 111: 267–274, 2003. doi: 10.1097/01.PRS.0000033066.12439.C0. [DOI] [PubMed] [Google Scholar]
- 17. Fensterheim BA, Young JD, Luan L, Kleinbard RR, Stothers CL, Patil NK, McAtee-Pereira AG, Guo Y, Trenary I, Hernandez A, Fults JB, Williams DL, Sherwood ER, Bohannon JK. The TLR4 agonist monophosphoryl lipid a drives broad resistance to infection via dynamic reprogramming of macrophage metabolism. J Immunol 200: 3777–3789, 2018. doi: 10.4049/jimmunol.1800085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Owen AM, Fults JB, Patil NK, Hernandez A, Bohannon JK. TLR agonists as mediators of trained immunity: mechanistic insight and immunotherapeutic potential to combat infection. Front Immunol 11: 622614, 2020. doi: 10.3389/fimmu.2020.622614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sherwood ER, Burelbach KR, McBride MA, Stothers CL, Owen AM, Hernandez A, Patil NK, Williams DL, Bohannon JK. Innate immune memory and the host response to infection. J Immunol 208: 785–792, 2022. doi: 10.4049/jimmunol.2101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, Joosten LAB, van der Meer JWM, Mhlanga MM, Mulder WJM, Riksen NP, Schlitzer A, Schultze JL, Stabell Benn C, Sun JC, Xavier RJ, Latz E. Defining trained immunity and its role in health and disease. Nat Rev Immunol 20: 375–388, 2020. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murphey ED, Fang G, Varma TK, Sherwood ER. Improved bacterial clearance and decreased mortality can be induced by LPS tolerance and is not dependent upon IFN-gamma. Shock 27: 289–295, 2007. doi: 10.1097/01.shk.0000245024.93740.28. [DOI] [PubMed] [Google Scholar]
- 22. Deng M, Scott MJ, Loughran P, Gibson G, Sodhi C, Watkins S, Hackam D, Billiar TR. Lipopolysaccharide clearance, bacterial clearance, and systemic inflammatory responses are regulated by cell type-specific functions of TLR4 during sepsis. J Immunol 190: 5152–5160, 2013. doi: 10.4049/jimmunol.1300496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bohannon JK, Luan L, Hernandez A, Afzal A, Guo Y, Patil NK, Fensterheim B, Sherwood ER. Role of G-CSF in monophosphoryl lipid A-mediated augmentation of neutrophil functions after burn injury. J Leukoc Biol 99: 629–640, 2016. doi: 10.1189/jlb.4A0815-362R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458: 1191–1195, 2009. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 25. Bohannon JK, Hernandez A, Enkhbaatar P, Adams WL, Sherwood ER. The immunobiology of Toll-like receptor 4 agonists: from endotoxin tolerance to immunoadjuvants. Shock 40: 451–462, 2013. doi: 10.1097/SHK.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hernandez A, Luan L, Stothers CL, Patil NK, Fults JB, Fensterheim BA, Guo Y, Wang J, Sherwood ER, Bohannon JK. Phosphorylated hexa-acyl disaccharides augment host resistance against common nosocomial pathogens. Crit Care Med 47: e930–e938, 2019. doi: 10.1097/CCM.0000000000003967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chiba T, Skrypnyk NI, Skvarca LB, Penchev R, Zhang KX, Rochon ER, Fall JL, Paueksakon P, Yang H, Alford CE, Roman BL, Zhang MZ, Harris R, Hukriede NA, de Caestecker MP. Retinoic acid signaling coordinates macrophage-dependent injury and repair after AKI. J Am Soc Nephrol 27: 495–508, 2016. doi: 10.1681/ASN.2014111108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Novitskaya T, McDermott L, Zhang KX, Chiba T, Paueksakon P, Hukriede NA, de Caestecker MP. A PTBA small molecule enhances recovery and reduces postinjury fibrosis after aristolochic acid-induced kidney injury. Am J Physiol Renal Physiol 306: F496–F504, 2014. doi: 10.1152/ajprenal.00534.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG, James JA, Salto-Tellez M, Hamilton PW. QuPath: open source software for digital pathology image analysis. Sci Rep 7: 16878, 2017. doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scarfe L, Menshikh A, Newton E, Zhu Y, Delgado R, Finney C, de Caestecker MP. Long-term outcomes in mouse models of ischemia-reperfusion-induced acute kidney injury. Am J Physiol Renal Physiol 317: F1068–F1080, 2019. doi: 10.1152/ajprenal.00305.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fu Y, Tang C, Cai J, Chen G, Zhang D, Dong Z. Rodent models of AKI-CKD transition. Am J Physiol Renal Physiol 315: F1098–F1106, 2018. doi: 10.1152/ajprenal.00199.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lever JM, Boddu R, George JF, Agarwal A. Heme oxygenase-1 in kidney health and disease. Antioxid Redox Signal 25: 165–183, 2016. doi: 10.1089/ars.2016.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 273: 4135–4142, 1998. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 34. Dase J, Rasyid H, Masadah R, Cangara MH, Bukhari A, Dwiyanti R, Hatta M. Analysis of mRNA and protein kidney injury molecule-1 (KIM-1) expression in a kidney model during the initiation phase of ischemia reperfusion injury. Ann Med Surg (Lond) 75: 103373, 2022. doi: 10.1016/j.amsu.2022.103373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jou-Valencia D, Koeze J, Popa ER, Aslan A, Zwiers PJ, Molema G, Zijlstra JG, van Meurs M, Moser J. Heterogenous renal injury biomarker production reveals human sepsis-associated acute kidney injury subtypes. Crit Care Explor 1: e0047, 2019. doi: 10.1097/CCE.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bonventre JV. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant 24: 3265–3268, 2009. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- 37. Lim AI, Tang SC, Lai KN, Leung JC. Kidney injury molecule-1: more than just an injury marker of tubular epithelial cells? J Cell Physiol 228: 917–924, 2013. doi: 10.1002/jcp.24267. [DOI] [PubMed] [Google Scholar]
- 38. Lim AI, Chan LY, Lai KN, Tang SC, Chow CW, Lam MF, Leung JC. Distinct role of matrix metalloproteinase-3 in kidney injury molecule-1 shedding by kidney proximal tubular epithelial cells. Int J Biochem Cell Biol 44: 1040–1050, 2012. doi: 10.1016/j.biocel.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 39. Arts RJW, Moorlag SJCFM, Novakovic B, Li Y, Wang SY, Oosting M, Kumar V, Xavier RJ, Wijmenga C, Joosten LAB, Reusken CBEM, Benn CS, Aaby P, Koopmans MP, Stunnenberg HG, van Crevel R, Netea MG. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 23: 89–100.e5, 2018. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 40. Mitroulis I, Ruppova K, Wang B, Chen LS, Grzybek M, Grinenko T, Eugster A, Troullinaki M, Palladini A, Kourtzelis I, Chatzigeorgiou A, Schlitzer A, Beyer M, Joosten LAB, Isermann B, Lesche M, Petzold A, Simons K, Henry I, Dahl A, Schultze JL, Wielockx B, Zamboni N, Mirtschink P, Coskun Ü, Hajishengallis G, Netea MG, Chavakis T. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172: 147–161.e12, 2018. doi: 10.1016/j.cell.2017.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1 and S2: https://doi.org/10.6084/m9.figshare.22191601.v1.
Data Availability Statement
No unique resources or reagents have been generated with this work. Original quantitative data will be provided by the authors on request.


