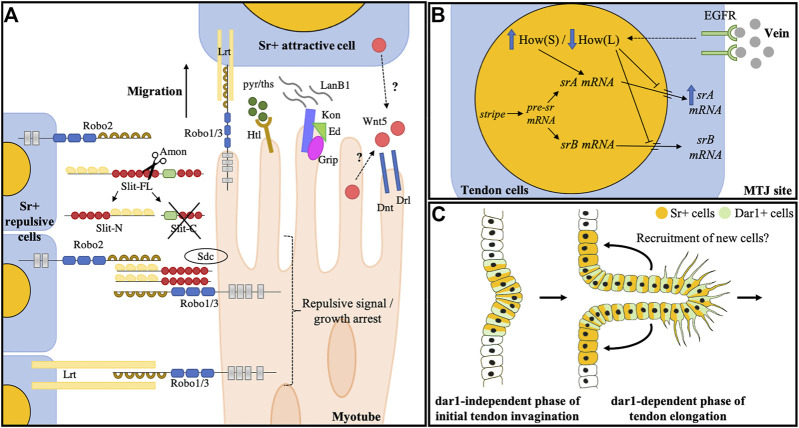
FIGURE 2.
Larval muscle guidance during embryogenesis and tendon development of leg muscles. (A) Known molecular actors controlling myotube guidance/arrest toward tendon cells during embryogenesis. Slit is cleaved by Amontillado protease (Ordan and Volk, 2016) into a rapidly degraded Slit-C fragment and a Slit-N that remains tethered to the membrane of the expressing cell by binding to the robo2 receptor. Slit-N is interpreted as a short-range repulsive and/or arrest signal by approaching myotubes through its interaction with Robo1/3 and Syndecan co-receptors (Chanana et al., 2009). Later, it was proposed that Robo1/3 functions in the muscle arrest through its interaction with the LRT protein. Other molecules also impact muscle guidance: the complex Kon/Ed/Grip accumulates at the tip of some myotubes and interacts with LanB1to promote their migration. Wnt5 protein is secreted by both muscle and tendon cells and acts through Drl and Dnt transmembrane receptors to regulate muscle migratory behavior. FGF signaling is also an essential regulator of myotube guidance through the regulation of cytoskeletal regulatory proteins. Figure is adapted from Ordan and Volk (2016). (B) Terminal differentiation of tendon larval muscles. Myotubes reaching their site of attachment release the vein ligand. Vein accumulates at the MTJ and binds to its receptor (EGFR) at the tendon cell membrane. EGF pathway activation triggers a switch between How (L) and How (S) isoforms. Both How isoforms can bind sr mRNA, How (L) isoform represses the sr mRNA nuclear export and leads to its degradation and How (S) favors the splicing of the srA isoform, leading to upregulation of the srA protein that triggers tendon terminal differentiation. (C) Hypothetical model depicting dar1 function in long tendon development of leg. dar1-independent phase: In the first phase of long tendon morphogenesis, Notch and odd are responsible for epithelium folding (i.e., invagination). During this phase, Notch is also responsible for initiating the tendon progenitor commitment by inducing stripe expression in a few epithelial cells (Laddada et al., 2019). Stripe then induces dar1 expression. dar1-dependent phase: During the second phase, dar1 regulates cytoskeleton remodeling and filopodia formation to promote collective cell migration and tendon elongation. In turn, the pulling mechanical forces may instigate the recruitment of new Sr-positive cells.