Abstract
Objective
The etiology of bipolar disorder (BD) is still not fully understood. Little is currently known about the relationship between the interaction the gastrointestinal system and brain function and BD. Zonulin is the only known physiological modulator of tight junctions and is a biomarker for intestinal permeability (IP). Occludin is an integral transmembrane tight junction protein involved in the maintenance and assembly of such junctions. The current study aims to determine whether zonulin and occludin levels are altered in BD and whether they can serve as clinical biomarkers of disease.
Methods
Forty-four patients with BD and 44 healthy controls were included in this study. The Young Mania Rating Scale (YMRS) was used to determine the severity of manic symptoms, while the Hamilton Depression Rating Scale (HDRS) was used to determine the severity of depressive symptoms, and the Brief Functioning Rating Scale (FAST) to assess functionality. Venous blood samples were taken from all participants and serum zonulin and occludin levels were measured.
Results
The mean serum zonulin and occludin levels of the patients were significantly higher compared to the healthy control group. There was no difference between manic, depressive, and euthymic patients in terms of zonulin and occludin levels. There was no correlation between the total number of attacks, duration of disease, YMRS, HDRS, FAST scores, and zonulin and occludin levels in the patient group. The groups were divided into three according to body mass index as normal, overweight, and obese. Zonulin and occludin levels increased as body mass index increased and were highest in the obese group.
Conclusion
The study shows that zonulin and occludin levels in BD increase independently of the disease stage. Consideration of the role of IP in the pathogenesis of BD may be helpful in determining the appropriate treatment modality.
Keywords: Bipolar disorder, Blood-brain barrier, Gastrointestinal tract, Gastrointestinal microbiota, Occludin, Zonulin
INTRODUCTION
Bipolar disorder (BD) is a chronic mental disorder progressing with recurrent attacks, that exhibits high mortality and morbidity levels, and that causes impairment in functionality in all areas [1]. An improved understanding of its neurobiology is needed to overcome obstacles such as its chronic course, the complexity of the diagnostic process, the uncertainty regarding its etiopathogenesis, and the limited effective therapeutic options [2].
The interaction between the gastrointestinal tract and brain functions has become an increasing topic of research in current psychiatric research. This versatile interaction occurs in the microbiota-gut-brain axis. The structure and mechanics of the blood-brain barrier (BBB) are in many ways like those of the intestinal barrier (IB) [3]. BBB permeability can be affected by factors such as psychological stress, oxidative stress, and proinflammatory cytokines. It is known that the same factors are disruptive for IB as well [4]. In recent years, many innovative studies have emphasized the possible connection between intestinal and BBB permeability and psychiatric disorders such as major depression, schizophrenia, obsessive compulsive disorder, autism spectrum disorder, and attention deficit hyperactivity disorder [5-10]. There is a very limited number of studies examining the relationship between BD, IB, and BBB permeability [11,12].
The intestinal epithelium has the largest contact area between the external environment and the human body. Therefore, it plays an extremely important role in maintaining immune homeostasis. IB is a single-layer epithelial structure composed of enterocytes. The adjoining lateral junctional complexes of cells are functionally classified as anchoring junctions (zonula adherence and macula adherence), tight junctions (zonula occludens), and gap junctions. Thanks to this complex protein system, selective permeability of the elements necessary for proper nutrition of the body is provided [13].
Zonulin is the only known physiological modulator of tight junctions [14]. Zonulin activates the epidermal growth factor receptor via proteinase-activated receptor 2. This leads to phosphorylation of tight junction proteins and rearrangement of actin filaments, which then causes suppression of tight junction proteins and ultimately increased intestinal permeability (IP) [15,16]. High zonulin levels indicate loss of control over particle passage from the intestinal lumen into the bloodstream, as well as IB destruction. Therefore, the evaluation of serum zonulin levels seems to be a good marker for the evaluation of IP. The discovery of zonulin, a protein that has regulatory activity on these tight junctions in the epithelium, has shed new light on its effect on the stability and maintenance of IB, and its role in causing several diseases in its dysfunction [17,18]. The importance of zonulin in maintaining IB tightness has been demonstrated in many studies [19]. Chronic inflammatory diseases with which zonulin is associated as an inflammatory marker include ankylosing spondylitis, celiac disease, chronic fatigue syndrome/myalgic encephalomyelitis, colitis, irritable bowel syndrome, hyperlipidemia, multiple sclerosis, necrotizing enterocolitis, non-alcoholic fatty liver disease, non-celiac gluten sensitivity, obesity, sepsis, and type 1 and 2 diabetes [20]. In addition, zonulin elevation has been linked to mental disorders such as attention deficit hyperactivity disorder, autism, schizophrenia, major depressive disorders, BD, obsessive compulsive disorder, and schizophrenia [9,10,12].
Occludin, an integral transmembrane tight junction protein, was first discovered in 1993. Data in studies on the function of occludin suggest that it plays a role in the maintenance and assembly of tight junctions. Occludin, in this role, is critically important in preventing the passage of materials from the intestinal lumen to the subepithelial tissue and from between cells to the lumen [21]. There is no study in the literature examining the relationship between BD and serum occludin level.
The current study aims to investigate whether the levels of circulating tight junction proteins zonulin and occludin differ between BD patients and healthy controls, and to determine whether zonulin and occludin can serve as clinical biomarkers in BD disease.
METHOD
Study group
The study was performed following approval from the Erzurum Regional Training And Research Hospital Ethical Committee (Erzurum BEAH KAEK 2021/06-126). In this study, 44 patients aged 18–65 years, who applied to our outpatient center or emergency department between April 2021 and April 2022, were evaluated as BD by applying the structured clinical interview for DSM-5-disorders-clinician version (SCID-5/CV). Informed consent was obtained from either them and/or their first-degree relatives; a total of 44 healthy volunteers were enrolled in the control group. The control group had come for a health check-up—a comprehensive health examination in the outpatient department. The control and patient groups were similar in terms of sociodemographic characteristics (age, gender, marital status, education, and employment status). In both the case and control groups, pregnant women, those with acute or chronic inflammation or inflammatory disease, those with a history of head trauma, and those with neurological, metabolic, or systemic diseases were excluded. A comprehensive diagnostic evaluation was made by a psychiatrist for each patient using Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) criteria, and those with comorbid psychiatric diseases were excluded from the study.
Tools
SCID-5/CV
This version was used to investigate axis I psychiatric disorders in cases according to DSM-5. It was developed by First et al. [22] and is a semi-structured interview guide for making major DSM-5 diagnoses. The validity and reliability study in Turkish was done by Elbir et al. [23]
Sociodemographic data form
It was used to investigate the sociodemographic, historical, and clinical characteristics of the cases.
Young Mania Rating Scale
Young Mania Rating Scale (YMRS) was used to confirm the severity of manic symptoms and well-being during the recovery period. This scale, completed by the interviewer, was developed by Young et al. [24], and its Turkish validity and reliability study was performed by Karadağ et al. [25]
Hamilton Depression Rating Scale
Hamilton Depression Rating Scale (HDRS) was used to confirm the severity of depressive symptoms and well-being during the recovery period. This scale, filled in by the interviewer, is a scale developed by Hamilton [26] and its validity and reliability in Turkish was confirmedby Akdemir et al. [27]
Brief Functioning Assessment Scale
Brief Functioning Assessment Scale (FAST) was used to evaluate the functionality levels of the cases. This scale, filled in by the interviewer, was developed by Rosa et al. [28], and adapted to Turkish by Aydemir and Uykur [29].
Application
The necessary permission to carry out this study was obtained from the Ethics Committee of the Health Sciences University Erzurum Regional Training and Research Hospital. SCID-5/CV, YMRS, HDRS, and FAST were applied to the cases.
For biochemical analysis, venous blood samples were taken from the antecubital region between 08:00 and 10:00 in the morning after the subjects and controls fasted overnight (12 hr). The collected venous blood was centrifuged at 4,500 rpm for 7 min in biochemistry tubes, and the serum sample obtained by separating the serum portions. These were then stored in Atatürk University Biochemistry laboratory at -80°C until the day of use.
Zonulin and occludin levels were measured with the ELISA method using a Human Zonulin ELISA Kit (Catalog No. EEL-H5560; Elabscience Biotechnology Inc., Houston, TX, USA) and a Human Occludin ELISA Kit (Catalog No. E-ELH1073; Elabscience Biotechnology Inc.) in line with the manufacturer’s instructions [7-10]. Kit measurement ranges for zonulin and occludin were 0.78–50 ng/mL and 0.16–10 ng/mL, respectively. The intra-assay coefficient of variation (CV) was 4.9%, with an inter-assay CV of 5.2% for zonulin. The intraassay CV was 4.8%, with an inter-assay CV of 4.8% for occludin. The biochemical analyzes of the cases were covered by the researchers’ own budget.
Statistical analysis
The SPSS 20.0 for Windows software (IBM Corp., Armonk, NY, USA) was employed for data recording and statistical analysis. Descriptive statistics were expressed as number and percentage for categorical variables and as mean±standard deviation for numerical variables. Normality of distribution was assessed using the Kolmogorov Smirnov test. One-way analysis of variance and the unpaired Student’s t-test were used to compare study groups, and the degree of significance between groups was determined using the Tukey post-hoc test. Also repeated measures analysis of covariance (ANCOVA) were used to investigate the effect of BMI as covariates. Statistical analysis of the difference between the groups in terms of gender distribution was performed with the χ2 test. The receiver operating characteristic curve, an expression of a specific method’s predictive power, was used to calculate zonulin and occludin sensitivity, specificity, area under the curve (AUC), and cut-off values. A value of p<0.05 was regarded as statistically significant.
RESULTS
Introduction of the sample
Sociodemographic and clinical findings of the groups are given in Table 1. There was no statistically significant difference between the groups in terms of gender distribution and mean age (p=0.67, p=0.88, respectively) (Table 1).
Table 1.
Demographic and clinical characteristics of BD patients and control group members
| Characteristics | Bipolar disorder (N=44) | Control group (N=44) | p |
|---|---|---|---|
| Age (yr) | 40.8±13.2 | 41.2±13.1 | 0.88 |
| Gender, male/female | 26/18 | 24/20 | 0.67 |
| Marital status, single/married/seperated | 17/26/1 | 15/27/2 | 0.56 |
| Educational background, primary school/secondary school and above | 27/17 | 30/14 | 0.51 |
| Employment status, notworking/working/female homemaker/student | 14/11/16/3 | 13/11/17/3 | 0.83 |
| Alcohol use, yes/no | 12/32 | 3/41 | 0.01 |
| Smoking, yes/no | 29/15 | 18/26 | 0.02 |
| Drug use, yes/no | 4/40 | 1/43 | 0.17 |
| BMI (kg/m2) | 28.3±3.9 | 22.6±3.3 | <0.001 |
| Disease onset age (yr) | 26.3±10.9 | - | - |
| Disease duration (yr) | 13.1±9.8 | - | - |
| Number of attacks | 7.9±5.2 | - | - |
| History of attempted suicide, yes/no | 7/37 | - | - |
| YMRS | 18.6±18.5 | - | - |
| HDRS | 3.6±8.6 | - | - |
| FAST | 35.8±17.1 | - | - |
Values are presented as mean±standard deviation or number. BD, bipolar disorder; BMI, body mass index; YMRS, Young Mania Rating Scale; HDRS, Hamilton Depression Rating Scale; FAST, Brief Functioning Assessment Scale
As indicated in Table 1, there was no significant difference between the groups for education level, marital status, employment status, and drug and/or stimulant use (p=0.51, 0.56, 0.83, and 0.17, respectively). However, a significant difference was determined in terms of alcohol and cigarette use (p=0.01, p=0.02, respectively).
The groups were divided into three according to body mass index (BMI) as normal, overweight, and obese. Zonulin and occludin levels increased as the BMI increased, with these levels highest in the obese group. In the pairwise comparison of the groups, there was a significant difference in terms of zonulin and occludin levels. However, when the occludin levels of the normal and overweight groups were compared, no significant difference was found (Table 2).
Table 2.
Zonulin and occludin levels in groups separated according to body mass index
| Normal | Overweight | Obese | p | |
|---|---|---|---|---|
| Zonulin | 113.7±23.5 | 156.7±24.9 | 200.1±21.6 | <0.001*†‡ |
| Occludin | 1.5±1.1 | 2.4±1.6 | 4.3±1.8 | 0.28* |
| 0.01† | ||||
| 0.03‡ |
Values are presented as mean±standard deviation.
between normal and overweight;
between normal and obesity;
between overweight and obesity
Clinical characteristics of the BD group
Within the study, the number of patients with bipolar 1 disorder was 34 (86.4%) and the number of patients with bipolar 2 disorder was 6 (3.6%). Seven of the patients (15.9%) had a history of attempted suicide. Disease onset age was 26 (26.3± 10.9) years, total disease duration was 13 (13.1±9.8) years, and the number of episodes was 8 (7.9±5.2). When the patients’ drug use was examined, it was determined that all subjects were using drug therapy, all subjects were using mood stabilizers, 3 (6.8%) cases were using only one mood stabilizer, and the other 41 (93.2%) cases were using antidepressants and/or antipsychotics in addition to mood stabilizers.
When the scale scores of the patients were examined, the YMRS score was 18.6±18.5, the HDRS score was 3.59±8.6, and the FAST score was 35.8±17.1. There was no correlation between zonulin or occludin levels and YMRS scores, HDRS scores, and FAST scores in the patient group (p>0.05). Again, there was no correlation between the total number of attacks and disease duration and zonulin and occludin levels in the patient group (p>0.05).
Zonulin and occludin levels of the groups
The zonulin and occludin levels of all groups are given in Table 3. Zonulin and occludin levels were found to be significantly higherin patients compared to those in the control group (p<0.001). Also in an ANCOVA taking baseline BMI as covariance the difference between groups remained significant (p<0.001 for both zonulin and occludin).
Table 3.
Zonulin and occludin levels of the patient and control groups
| Bipolar disorder | Control group | p | |
|---|---|---|---|
| Zonulin | 170.0±34.8 | 117.2±31.6 | <0.001 |
| Occludin | 2.7±1.6 | 1.1±0.6 | <0.001 |
Values are presented as mean±standard deviation
Zonulin and occludin levels by disease period
The cases were divided into 3 groups according to their YMRS and HDRS scores as manic (n=21), depressive (n=7), and euthymic (n=16). The zonulin level was 171.1± 38.4 ng/mL in the manic group, 166.3±38.5 ng/mL in the depressive group, and 170.2±29.9 ng/mL in the euthymic group, while the occludin level was 2.6±1.5 ng/mL in the manic group, 2.5±1.4 ng/mL in the depressive group, and 2.9±1.7 ng/mL in the euthymic group. There was no significant difference between the groups in terms of zonulin and occludin levels (p=0.95, p=0.85, respectively) (Table 4). In addition, there was no significant difference in drug use between the 3 groups separated according to disease stage (p=0.49).
Table 4.
Serum zonulin and occludin levels in the bipolar disorder group
| Manic (N=21) | Depressive (N=7) | Euthymic (N=16) | p | |
|---|---|---|---|---|
| Zonulin | 171.1±38.4 | 166.3±38.5 | 170.2±29.9 | 0.95 |
| Occludin | 2.6±1.5 | 2.5±1.4 | 2.9±1.7 | 0.85 |
Values are presented as mean±standard deviation
When we divided the patients into 2 groups according to suicide attempt, the zonulin levels of those who committed suicide (n=7) were found to be 171.1±35.2 and 164.0±34.9 of those who did not. Occludin levels are 2.7±1.6 in those who commit suicide and 2.6±1.4 in those who do not. Although zonulin and occludin levels were higher in those who committed suicide, no statistically significant difference was found between the groups (p=0.63, p=0.90, respectively).
When patients were divided into two according to suicide attempts, zonulin levels were found to be 171.1±35.2 in those who had attempted to commit suicide (n=7) and 164.0±34.9 in those who did not. Occludin levels were 2.7±1.6 in those who had attempted to commit suicide and 2.6±1.4 in those who had not. Although zonulin and occludin levels were higher in those who had attempted to commit suicide, there was no statistically significant difference between the groups (p=0.63, p=0.91, respectively).
Specificity and sensitivity
When the cutoff value was taken as 133.2 ng/mL, the sensitivity of serum zonulin levels in distinguishing BD patients from healthy individuals was 84%, and the specificity was 75% (AUC=0.87, p<0.001 at 95% confidence interval [0.79– 0.94]) (Figure 1).
Figure 1.
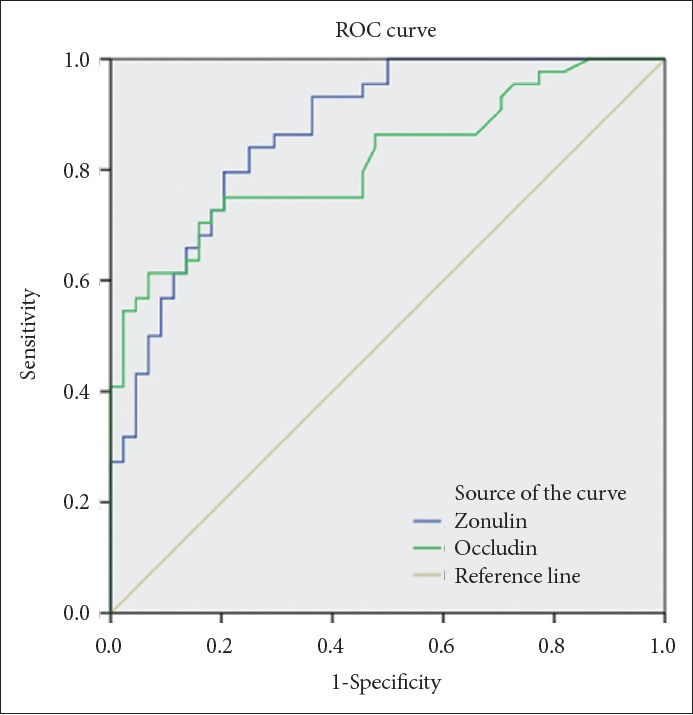
Determination of the diagnostic sensitivity and specificity of serum zonulin and occludin levels in patients with BD using ROC curve analysis. Diagonal segments are produced by ties. BD, bipolar disorder; ROC, receiver-operating characteristic curve.
When the cutoff value was taken as 1.4 ng/mL, the sensitivity of serum occludin levels in distinguishing BD patients from healthy individuals was 75% and the specificity was 80% (AUC=0.82, p<0.001 at 95% confidence interval [0.73–0.91]) (Figure 1).
DISCUSSION
In this study, which was conducted to understand whether serum zonulin and occludin levels in BD patients were different compared to the those in the healthy control group and whether they could serve as clinical biomarkers of disease, zonulin and occludin levels were found to be significantly higher in BD patients compared to those in the control group. This result is consistent with our hypothesis. It was previously suggested by Kılıç et al. [30] that zonulin and claudin-5 levels are higher in BD patients than in the healthy control group, and this finding indicates the role of intestinal and BBB permeability in the pathogenesis of BD. In another recent study, it was reported that plasma zonulin levels did not change in the bipolar 1 disorder group compared to those in the healthy control group. When we look at the literature, there is no other report with which we can compare our findings, in reference to the relationship between occludin levels and BD.
Zonulin is a modulator of epithelial and endothelial barrier functions. In intestinal dysbiosis, zonulin is released from the barrier into the circulation with epithelial disruption [31]. There are other reports in the literature that high serum levels of zonulin and occludin indicate IB destruction [32]. With IB disruption, the passage of luminal contents across the epithelial barrier is followed by the passage of microbial antigens that trigger T cell activation. In this case, there is a release of proinflammatory cytokines and a further increase in permeability. This event occurs as a vicious circle [20]. Clinical studies have shown that zonulin may be responsible for the breakdown of both the IB and the BBB in intestinal dysbiosis [33]. Proinflammatory cytokines can both directly affect the cells in the brain by crossing the BBB easily and affect neurotransmitter metabolism and the pituitary-hypothalamus and adrenal axis [34]. The effect of proinflammatory cytokines in both the depressive and manic periods of BD has been shown in many studies [35]. Kamintsky et al. [36] stated that BBB disruption may have an important clinical effect in BD patients and concluded that biomolecules such as zonulin, Claudin-5, and S100B, which play a role in maintaining BBB integrity, can be used as biomarkers to detect BBB deterioration. In light of this information, the higher zonulin and occludin levels in the patient group in the present study are consistent with the evidence for their role in BD. In addition, parallel zonulin and occludin elevations suggest that they may have similar effects on IP.
In the current study, when the BD group was divided into manic, depressive, and euthymic, no significant difference was found between the groups in terms of zonulin and occludin levels. Aydın et al. [12], in their study, reported that there was no difference in zonulin levels in the bipolar 1 disorder group during illness, exacerbation and remission periods, and there was no significant relationship between disease symptoms and zonulin levels. The absence of a relationship between different disease stages of BD and zonulin and occludin levels in the current study supports this finding. A possible interpretation, therefore, is that zonulin and occludin levels increase independently of the disease period.
In this study, although zonulin and occludin levels were higher in cases with a history of attempted suicide, no statistically significant relationship was found between the groups. In the literature, there are studies examining the relationship between suicidal behavior and IP. Ohlsson et al. [37], in their study, compared the levels of IP markers in subjects with a recent suicide attempt, major depressive disorder patients without a history of suicide attempts, and healthy controls. They found the lowest zonulin level and higher intestinal fatty acid binding protein level in the group who had recently attempted suicide. They found no significant difference in soluble CD14 levels between the groups and correlated this result with the diagnostic heterogeneity of the group who had recently attempted suicide. In the current study, the relatively low number of patients, who attempted suicide, may be a reason why no significant relationship was found with zonulin and occludin in patients with a history of attempted suicide.
A high-fat diet disrupts IB integrity and produces behavioral changes consistent with depression and anxiety [38]. In this study, in order to evaluate the effects of a high-fat diet on IP, BMI was measured in our groups, and when the groups were divided into three as normal, overweight, and obese during the evaluation phase, it was found that zonulin and occludin levels increased as BMI increased. This result shows the effect of nutrition on IP and the findings are compatible with those in the literature [39,40].
The majority of drugs used to treat BD need to interact with the BBB in order to exhibit their effect. BBB dysfunction is important for the effectiveness of treatment. In studies, successful treatment of attacks in BD was associated with a decrease in markers linked to BBB deterioration [41]. Significantly decreased hippocampal BBB permeability has been reported in rats exposed to lithium, while lithium treatment increases the expression of hippocampal claudin-5 and brain-derived neurotrophic factor protein [42-44]. A decrease in proinflammatory molecule levels and an increase in occludin and claudin-5 levels has been reported in mice treated with valproic acid [11]. Low antipsychotic drug doses have been linked to neuroprotection in the majority of studies, while higher doses have been associated with cytotoxic effects on BBB endothelial cells [45,46]. Studies have shown that antidepressants interact with proteins on the BBB epithelial surface. In addition, clinically successful antidepressant therapy has been associated with decreases in S100B levels, which are known to rise in BD and to be linked to BBB impairment [47,48].
In this study, the use of mood stabilizers by all subjects and the use of antidepressants in some cases and antipsychotics in addition to mood stabilizers did not allow for anevaluation of the relationship between zonulin and occludin levels and pharmacological agents.
Limitations and advantages of the study
The first limitation of this study is its cross-sectional nature. Longitudinal monitoring studies would facilitate a better understanding and interpretation of the findings. For this reason, it is appropriate to repeat similar studies longitudinally. The number of patients makes it difficult to generalize because it is necessary to work with borderline numbers for statistical evaluation in subgroup comparisons. It would be possible to overcome this limitation by working with larger groups. In addition, the absence of patients, who did not use drugs, is a limitation in our study, but it is thought that future studies can overcome this limitation.
Among the advantages of the study is the use of individuals with similar characteristics to the patient group instead of the use of a random control group. The fact that the cases included depressive, manic, and euthymic episodes of BD makes this study powerful. Another advantage of the study is that BMI, which affects IP increase, is taken into consideration and individuals with diseases progressing with IP increase are not included in the study.
As a result of this study, it was revealed that serum zonulin and occludin levels in BD are significantly higher regardless of the disease stage. Consideration of the role of IP in the pathogenesis of BD may be helpful in determining an appropriate treatment modality. More experimental and clinical research is needed to investigate the role of circulating zonulin and occludin levels in BD. The data in this study should be considered preliminary until replicated in larger cohorts.
Acknowledgments
We would like to thank the patients who participated in this study.
Footnotes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Sertaç Zengil, Esra Laloğlu. Data curation: Sertaç Zengil, Esra Laloğlu. Formal analysis: Sertaç Zengil, Esra Laloğlu. Methodology: Sertaç Zengil. Supervision: Esra Laloğlu. Wiriting—original draft: Sertaç Zengil. Writing—review & editing: Sertaç Zengil, Esra Laloğlu.
Funding Statement
None
REFERENCES
- 1.Smith DJ, Whitham EA, Ghaemi SN. Bipolar disorder. Handb Clin Neurol. 2012;106:251–263. doi: 10.1016/B978-0-444-52002-9.00015-2. [DOI] [PubMed] [Google Scholar]
- 2.Beyer DKE, Freund N. Animal models for bipolar disorder: from bedside to the cage. Int J Bipolar Disord. 2017;5:35. doi: 10.1186/s40345-017-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daneman R, Rescigno M. The gut immune barrier and the blood-brain barrier: are they so different? Immunity. 2009;31:722–735. doi: 10.1016/j.immuni.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Rudzki L, Szulc A. “Immune gate” of psychopathology—The role of gut derived immune activation in major psychiatric disorders. Front Psychiatry. 2018;9:205. doi: 10.3389/fpsyt.2018.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett. 2008;29:117–124. [PubMed] [Google Scholar]
- 6.Maes M, Kubera M, Leunis JC, Berk M. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J Affect Disord. 2012;141:55–62. doi: 10.1016/j.jad.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Usta A, Kılıç F, Demirdaş A, Işık Ü, Doğuç DK, Bozkurt M. Serum zonulin and claudin-5 levels in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2021;271:767–773. doi: 10.1007/s00406-020-01152-9. [DOI] [PubMed] [Google Scholar]
- 8.Işık Ü, Aydoğan Avşar P, Aktepe E, Doğuç DK, Kılıç F, Büyükbayram Hİ. Serum zonulin and claudin-5 levels in children with obsessivecompulsive disorder. Nord J Psychiatry. 2020;74:346–351. doi: 10.1080/08039488.2020.1715474. [DOI] [PubMed] [Google Scholar]
- 9.Esnafoglu E, Cırrık S, Ayyıldız SN, Erdil A, Ertürk EY, Daglı A, et al. Increased serum zonulin levels as an intestinal permeability marker in autistic subjects. J Pediatr. 2017;188:240–244. doi: 10.1016/j.jpeds.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Özyurt G, Öztürk Y, Appak YÇ, Arslan FD, Baran M, Karakoyun İ, et al. Increased zonulin is associated with hyperactivity and social dysfunctions in children with attention deficit hyperactivity disorder. Compr Psychiatry. 2018;87:138–142. doi: 10.1016/j.comppsych.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Zhao NO, Topolski N, Tusconi M, Salarda EM, Busby CW, Lima CNNC, et al. Blood-brain barrier dysfunction in bipolar disorder: molecular mechanisms and clinical implications. Brain Behav Immun Health. 2022;21:100441. doi: 10.1016/j.bbih.2022.100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aydın O, Kocabaş T, Sarandöl A, Taştan İ, Onur E, Aydemir Ö, et al. Examination of plasma zonulin levels in bipolar I disorder: a case-control study with follow-up. J Neural Transm (Vienna) 2020;127:1419–1426. doi: 10.1007/s00702-020-02234-7. [DOI] [PubMed] [Google Scholar]
- 13.Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124:3–20. doi: 10.1016/j.jaci.2009.05.038. quiz: 21-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci. 2000;113:4435–4440. doi: 10.1242/jcs.113.24.4435. [DOI] [PubMed] [Google Scholar]
- 15.Vanuytsel T, Vermeire S, Cleynen I. The role of haptoglobin and its related protein, zonulin, in inflammatory bowel disease. Tissue Barriers. 2013;1:e27321. doi: 10.4161/tisb.27321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fasano A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin Gastroenterol Hepatol. 2012;10:1096–1100. doi: 10.1016/j.cgh.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serek P, Oleksy-Wawrzyniak M. The effect of bacterial infections, probiotics and zonulin on intestinal barrier integrity. Int J Mol Sci. 2021;22:11359. doi: 10.3390/ijms222111359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caviglia GP, Rosso C, Ribaldone DG, Dughera F, Fagoonee S, Astegiano M, et al. Physiopathology of intestinal barrier and the role of zonulin. Minerva Biotecnol. 2019;31:83–92. [Google Scholar]
- 19.Al-Ayadhi L, Zayed N, Bhat RS, Moubayed NMS, Al-Muammar MN, El-Ansary A. The use of biomarkers associated with leaky gut as a diagnostic tool for early intervention in autism spectrum disorder: a systematic review. Gut Pathog. 2021;13:54. doi: 10.1186/s13099-021-00448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. 2020;9:F1000. doi: 10.12688/f1000research.20510.1. Rev-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv Drug Deliv Rev. 2005;57:883–917. doi: 10.1016/j.addr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 22.First MB, Williams JB, Karg RS, Spitzer RL. User’s guide for the SCID-5-CV structured clinical interview for DSM-5® disorders: clinical version. Washington, DC: American Psychiatric Publishing, Inc; 2016. [Google Scholar]
- 23.Elbir M, Alp Topbaş Ö, Bayad S, Kocabaş T, Topak OZ, Çetin Ş, et al. [Adaptation and reliability of the structured clinical interview for DSM- 5-disorders - clinician version (SCID-5/CV) to the Turkish language] Turk Psikiyatri Derg. 2019;30:51–56. Turkish. [PubMed] [Google Scholar]
- 24.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 25.Karadağ F, Oral ET, Aran Yalçın F, Erten E. [The validity and reliability of the Young Mania Rating Scale in Turkey] Turkish J Psychiatry. 2021;13:107–114. Turkish. [PubMed] [Google Scholar]
- 26.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akdemir A, Örsel S, Dag i, Türkçapar H, İşcan N, Özbay H. [Clinical use and the reliability and validity of the Turkish version of the Hamilton Depression Rating Scale (HDRS)] J Psychiatry Psychol Psychopharmacol. 1996;4:251–259. doi: 10.1053/comp.2001.19756. Turkish. [DOI] [PubMed] [Google Scholar]
- 28.Rosa AR, Sánchez-Moreno J, Martínez-Aran A, Salamero M, Torrent C, Reinares M, et al. Validity and reliability of the functioning assessment short test (FAST) in bipolar disorder. Clin Pract Epidemiol Ment Health. 2007;3:5. doi: 10.1186/1745-0179-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aydemir Ö, Uykur B. [Reliability and validity study of the Turkish version of Functioning Assessment Short Test] Turkish J Psychiatry. 2012;23:193–200. Turkish. [PubMed] [Google Scholar]
- 30.Kılıç F, Işık Ü, Demirdaş A, Doğuç DK, Bozkurt M. Serum zonulin and claudin-5 levels in patients with bipolar disorder. J Affect Disord. 2020;266:37–42. doi: 10.1016/j.jad.2020.01.117. [DOI] [PubMed] [Google Scholar]
- 31.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91:151–175. doi: 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- 32.Lochhead JJ, Yang J, Ronaldson PT, Davis TP. Structure, function, and regulation of the blood-brain barrier tight junction in central nervous system disorders. Front Physiol. 2020;11:914. doi: 10.3389/fphys.2020.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camara-Lemarroy CR, Silva C, Greenfield J, Liu WQ, Metz LM, Yong VW. Biomarkers of intestinal barrier function in multiple sclerosis are associated with disease activity. Mult Scler. 2020;26:1340–1350. doi: 10.1177/1352458519863133. [DOI] [PubMed] [Google Scholar]
- 34.Schachter J, Martel J, Lin CS, Chang CJ, Wu TR, Lu CC, et al. Effects of obesity on depression: a role for inflammation and the gut microbiota. Brain Behav Immun. 2018;69:1–8. doi: 10.1016/j.bbi.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 35.Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21:1696–1709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamintsky L, Cairns KA, Veksler R, Bowen C, Beyea SD, Friedman A, et al. Blood-brain barrier imaging as a potential biomarker for bipolar disorder progression. Neuroimage Clin. 2020;26:102049. doi: 10.1016/j.nicl.2019.102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohlsson L, Gustafsson A, Lavant E, Suneson K, Brundin L, Westrin Å, et al. Leaky gut biomarkers in depression and suicidal behavior. Acta Psychiatr Scand. 2019;139:185–193. doi: 10.1111/acps.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seguella L, Pesce M, Capuano R, Casano F, Pesce M, Corpetti C, et al. High-fat diet impairs duodenal barrier function and elicits glia-dependent changes along the gut-brain axis that are required for anxiogenic and depressive-like behaviors. J Neuroinflammation. 2021;18:115. doi: 10.1186/s12974-021-02164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molina-Vega M, Castellano-Castillo D, Sánchez-Alcoholado L, Plaza-Andrade I, Perera-Martin G, Cabrera-Mulero A, et al. Relationship of zonulin with serum PCSK9 levels after a high fat load in a population of obese subjects. Biomolecules. 2020;10:748. doi: 10.3390/biom10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Küme T, Acar S, Tuhan H, Çatlı G, Anık A, Gürsoy Çalan Ö, et al. The relationship between serum zonulin level and clinical and laboratory parameters of childhood obesity. J Clin Res Pediatr Endocrinol. 2017;9:31–38. doi: 10.4274/jcrpe.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai MC, Huang TL. Decreased S100B serum levels after treatment in bipolar patients in a manic phase. Compr Psychiatry. 2017;74:27–34. doi: 10.1016/j.comppsych.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Taler M, Aronovich R, Henry Hornfeld S, Dar S, Sasson E, Weizman A, et al. Regulatory effect of lithium on hippocampal blood-brain barrier integrity in a rat model of depressive-like behavior. Bipolar Disord. 2021;23:55–65. doi: 10.1111/bdi.12962. [DOI] [PubMed] [Google Scholar]
- 43.Luo H, Chevillard L, Bellivier F, Mégarbane B, Etain B, Cisternino S, et al. The role of brain barriers in the neurokinetics and pharmacodynamics of lithium. Pharmacol Res. 2021;166:105480. doi: 10.1016/j.phrs.2021.105480. [DOI] [PubMed] [Google Scholar]
- 44.Newman SA, Pan Y, Short JL, Nicolazzo JA. Assessing the impact of lithium chloride on the expression of P-glycoprotein at the blood-brain barrier. J Pharm Sci. 2017;106:2625–2631. doi: 10.1016/j.xphs.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 45.Elmorsy E, Elzalabany LM, Elsheikha HM, Smith PA. Adverse effects of antipsychotics on micro-vascular endothelial cells of the human bloodbrain barrier. Brain Res. 2014;1583:255–268. doi: 10.1016/j.brainres.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt AJ, Krieg JC, Clement HW, Hemmeter UM, Schulz E, Vedder H, et al. Effects of quetiapine, risperidone, 9-hydroxyrisperidone and ziprasidone on the survival of human neuronal and immune cells in vitro. J Psychopharmacol. 2010;24:349–354. doi: 10.1177/0269881108096506. [DOI] [PubMed] [Google Scholar]
- 47.O’Brien FE, Dinan TG, Griffin BT, Cryan JF. Interactions between antidepressants and P-glycoprotein at the blood-brain barrier: clinical significance of in vitro and in vivo findings. Br J Pharmacol. 2012;165:289–312. doi: 10.1111/j.1476-5381.2011.01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schroeter ML, Abdul-Khaliq H, Krebs M, Diefenbacher A, Blasig IE. Serum markers support disease-specific glial pathology in major depression. J Affect Disord. 2008;111:271–280. doi: 10.1016/j.jad.2008.03.005. [DOI] [PubMed] [Google Scholar]


