Figure 1.
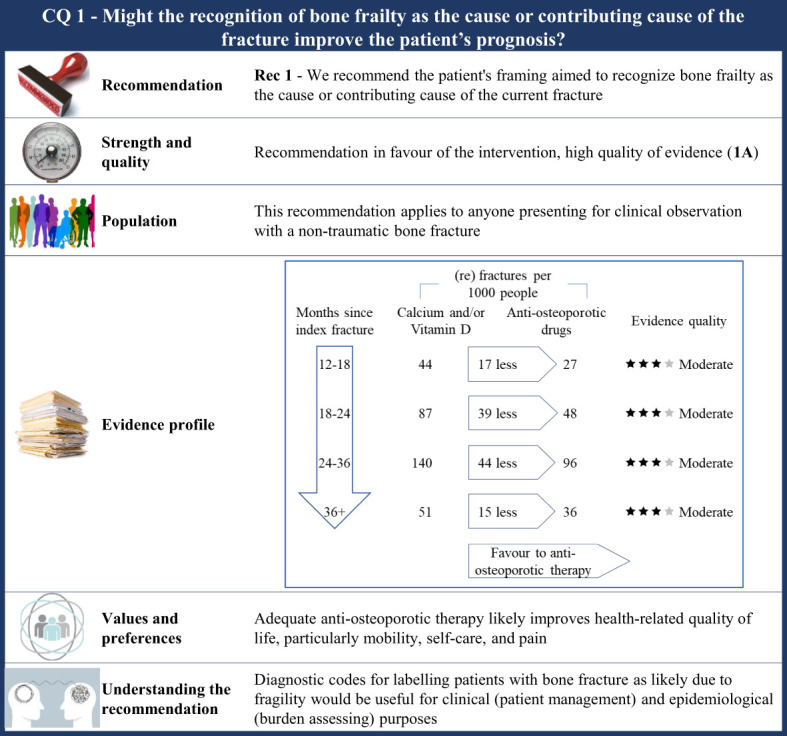
Visual summary for CQ1 (Might the recognition of frailty as the cause or contributing cause of the fracture improve the patient’s prognosis)?. Rationale. As ethical concerns hinder carrying out clinical studies by randomizing patients to obtain an adequate comparator (i.e., patients who have no tools to recognize bone frailty were included), the CQ was indirectly investigated. RCTs comparing outcome occurrence (refracture) among patients who received any anti-osteoporotic drug therapy and those who received calcium and/or vitamin D were included. The underlying assumption is that all the included patients were indicated for anti-osteoporotic drug therapy (i.e., the bone frailty was the cause or contributing cause of the current fracture), but some of them did not receive effective drug therapy (so surrogating those patients for whom no tools recognizing bone frailty are used, i.e., the comparator of interest). Through the updating of the most recently published systematic review on this issue (42), our systematic review included 46 RCTs (59–74, 89–104). Critical outcomes of interest pertained the rate of refracture at 12–18 months, 18–24 months, 24–36 months, and 3 years or more from the index fracture. Clinical benefits. Although the quality of evidence was moderate within each time category, a clear advantage favouring anti-osteoporotic drug therapy was observed. Between-rate absolute difference (RD) ranged from 15 to 44 (re)fractures avoided with therapy every 1,000 fractured patients, respectively, 36 months or more and 24–36 months after the index fracture. Values and preferences. Osteoporotic fractures have a negative impact on Health-related Quality of Life (HRQoL), particularly for mobility, self-care, and pain. Patients over 50 years of age treated with anti-osteoporotic therapy showed a significant improvement in HRQoL at 24 months (105). Increased quality of life as detected by the QUALIOST questionnaire was obtained through treatment of postmenopausal women (90), although no significant differences were found for the Short-Form or SF-36. At last, a higher Osteoporosis Quality of Life Scale score was reached after 12 months of drug therapy (99). Understanding the recommendation. The FFT noted that there were strong clinical benefits associated with anti-osteoporotic therapy and, consequently, agreed to upgrade up to high evidence quality despite the moderate certainty evidence. A combination of the evidence, values, and preferences also contributed to the strong recommendation in favour of the anti-osteoporotic treatment in patients with fragility fractures. Diagnostic codes for label patients who have bone fracture is likely due to fragility would be useful for clinic (patients’ managing) and epidemiologic (burden assessing) purposes.
