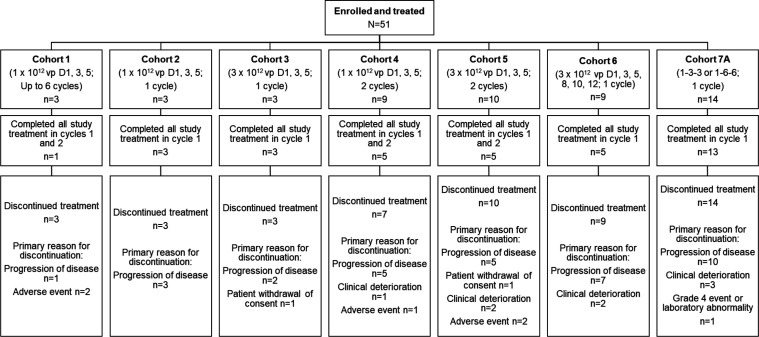
Figure 1.
Patient disposition. Patients in cohort 1 received enadenotucirev in combination with pembrolizumab. Patients in cohort 2 to cohort 7A received enadenotucirev in combination with nivolumab. Per protocol, the end of study was defined as when all patients had completed all study visits, had otherwise discontinued from the study, or the last patients treated in the study all had at least 9 months of follow-up after their first dose of study treatment. Since there was no set definition of the timing of study completion rates across all patients, study completion rates would not be a meaningful measure, nor appropriate to compare across cohorts, and therefore have not been provided. Instead, number of patients completing treatment is summarized in the figure. 1-3-3, 1×1012 vp on day 1 followed by 3×1012 vp on days 3 and 5; 1-6-6, 1×1012 vp on day 1 followed by 6×1012 vp on days 3 and 5.