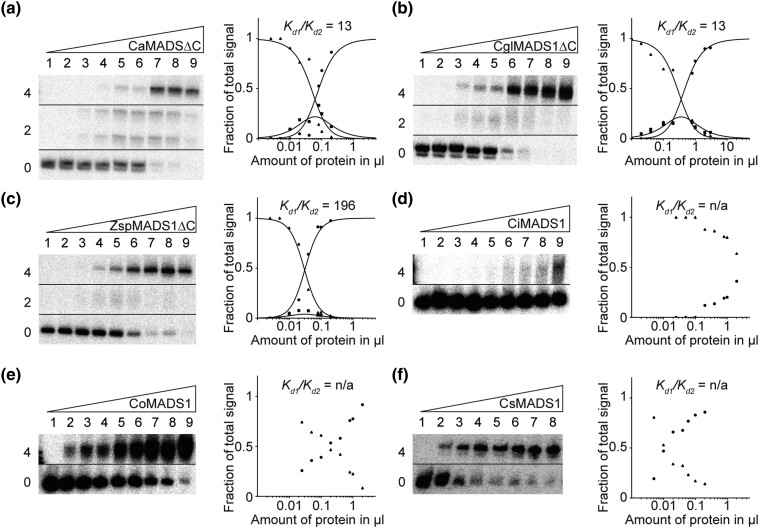
Fig. 6.
FQC formation capabilities of the charophyte MIKC-type proteins CaMADS1, CglMADS1, ZspMADS1, CiMADS1, CoMADS1, and CsMADS1. Increasing amounts of in vitro transcribed/translated (a) CaMADS1ΔC, (b) CglMADS1ΔC, (c) ZspMADS1ΔC, (d) CiMADS1, (e) CoMADS1, and (f) CsMADS1, were coincubated together with constant amounts of DNA probe 1. For details see legend of figure 1. Because (d) CiMADS1, (e) CoMADS1, and (f) CsMADS1 produced no signal of intermediate electrophoretic mobility constituting a DNA probe bound by a single protein dimer, Kd1/Kd2 cannot be determined. In case of (a) CaMADS1, a double band was observed for the fraction of intermediate electrophoretic mobility, likely caused by different conformations of the DNA-bound protein dimer. Because CaMADS1, CglMADS1, and ZspMADS1 full-length proteins comprise very long C-terminal domains, resulting in low DNA-binding affinities and blurry bands, C-terminally truncated mutants were used instead. Applied amounts of in vitro transcription/translation products were (a, c–e) (lanes 1–9) 0, 0.025, 0.05, 0.1, 0.2, 0.4, 0.8, 1, and 2 µl, whereby CaMADS1ΔC and ZspMADS1ΔC were prediluted 1:10 with BSA (10 mg/ml), (b) (lanes 1–9) 0.02, 0.04, 0.1, 0.2, 0.4, 0.8, 1, 2, and 3 µl, (f) (lanes 1–8) 0, 0.005, 0.01, 0.02, 0.04, 0.06, 0.1, and 0.2 µl.