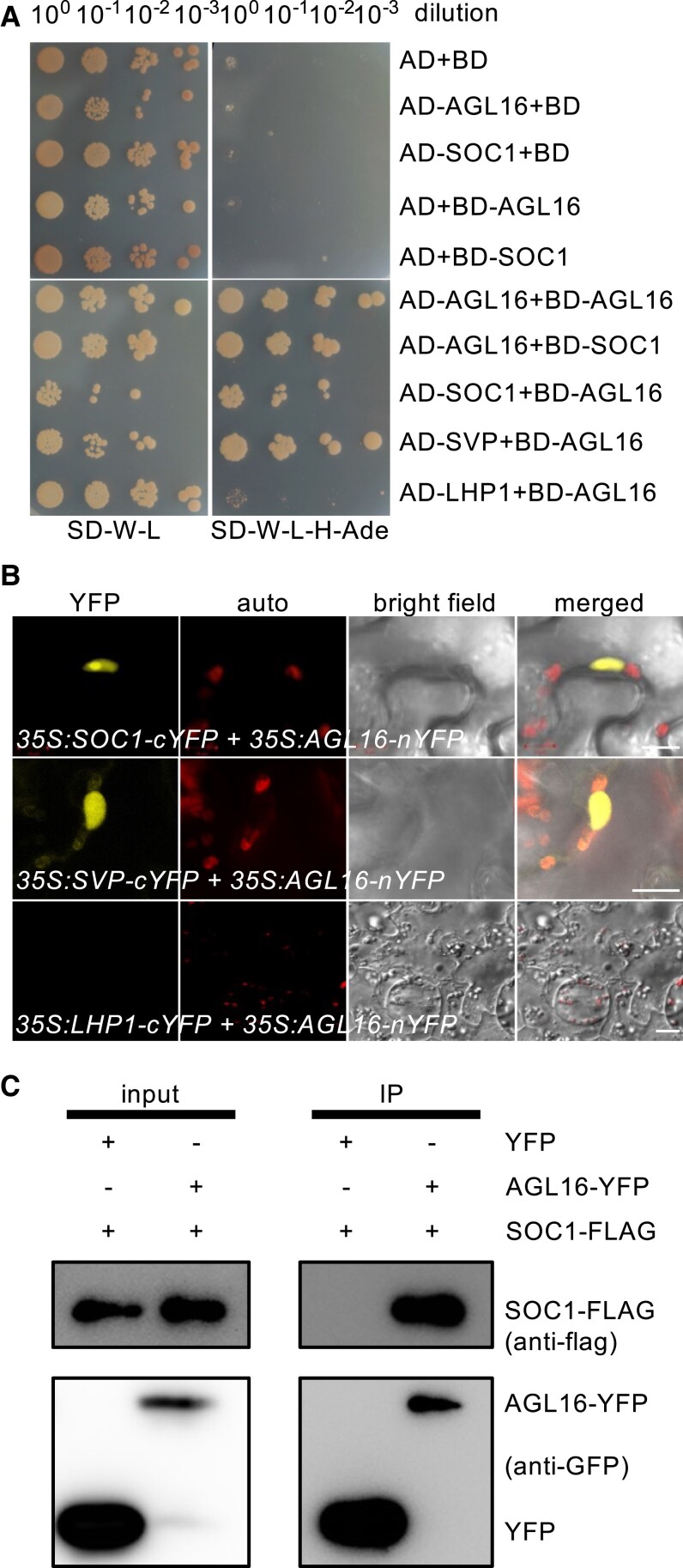
Figure 5.
AGL16 forms protein complex with SOC1. A) Yeast two-hybrid assay revealed a direct interaction between AGL16 and SOC1. Each protein was fused to either the activation domain (AD) as prey or the DNA-binding domain (BD) as bait. Serial dilutions (100x to 10−3x) of J69-4A cells containing different construct combinations indicated on the left were grown on control (left) and selective (right) medium. The AGL16-SVP and the AGL16-LHP1/empty vector combinations provided positive and negative controls, respectively. B) BiFC assay evidenced the formation of AGL16–SOC1 complex in nucleus of N. benthamiana leaf epidermis. The interaction was tested with constructs 35S:SOC1-cYFP and 35S:AGL16-nYFP. A negative interaction between AGL16 and LHP1 and a positive interaction between AGL16 and SVP were tested as well. Bars = 10 µm. C) Co-immunoprecipitation (co-IP) assay confirmed the AGL16–SOC1 interaction in Arabidopsis protoplast. SOC1 was FLAG-tagged while the AGL16 was fused with an YFP tag. Total protein of the transfected wild type protoplasts was immuno-precipitated with antibody against GFP (anti-GFP) first, and further analyzed by Western blot using antibody against FLAG (anti-FLAG).