Abstract
Childhood adversity is a leading transdiagnostic risk factor for psychopathology, being associated with an estimated 50–65% of childhood-onset disorders and 23–42% of adult-onset disorders (Kessler et al., 2010). Major unresolved theoretical challenges stem from the nonspecific and probabilistic nature of the links between childhood adversity and psychopathology. The links are nonspecific, because childhood adversity increases risk, through a range of mechanisms, for diverse forms of psychopathology, and are probabilistic, because not all individuals exposed to childhood adversity develop psychopathology. In this article, we propose a path forward by focusing on stress phenotypes, defined as biobehavioral patterns activated in response to stressors that can disrupt future functioning when persistent (e.g., reward seeking, social withdrawal, aggression). This review centers on the accumulating evidence that psychopathology appears to be more strongly predicted by behavior and biology during states of stress. Building on this observation, our theoretical framework proposes that we can model pathways from childhood adversity to psychopathology with greater specificity and certainty by understanding stress phenotypes, defined as patterns of behavior and their corresponding biological substrates that are elicited by stressors. This approach aims to advance our conceptualization of mediating pathways from childhood adversity to psychopathology. Understanding stress phenotypes will bring us closer to “precision mental health”, a person-centered approach to identifying, preventing, and treating psychopathology.
Keywords: childhood adversity, psychopathology, stress, phenotypes
General Scientific Summary:
Childhood adversity increases risk for numerous forms of psychopathology through diverse pathways. We propose that prediction and explanation of psychopathology based on childhood adversity can be improved by studying stress phenotypes. Stress phenotypes are defined as patterns of behavior and their corresponding biological substrates that are elicited under stress.
Childhood adversity, defined as exposure to environmental stressors before age 18, is a leading transdiagnostic risk factor for psychopathology. The World Health Organization (WHO) World Mental Health Survey showed that childhood adversities increase the risk of all classes of psychiatric disorders across the 21 countries sampled (Kessler et al., 2010). Population-attributable risk proportions have indicated that eliminating childhood adversities could reduce the prevalence of childhood-onset disorders by 50–65% and the prevalence of adult-onset disorders by 23–42%. Interventions, experiments, and natural quasi-experiments have demonstrated that childhood adversity plays a causal role in the development of psychopathology (e.g., Cicchetti et al., 2006; Costello et al., 2010; Dozier et al., 2018; Masten & Narayan, 2012; Morris et al., 2017; Wade et al., 2019). Despite the clear link between childhood adversity and psychopathology, questions about the myriad pathways explaining their association remain unresolved.
Although childhood adversity undoubtedly plays an integral role in the development of psychopathology, the nonspecific and probabilistic links between adversity and psychopathology raise two major theoretical challenges. The links are nonspecific because childhood adversity increases risk for diverse forms of psychopathology, including internalizing disorders, externalizing disorders, and personality disorders (Kessler et al., 2010; Vachon et al., 2015), and does so through various mechanisms. The links are also probabilistic because not all individuals exposed to childhood adversity develop psychopathology, a phenomenon known as resilience (Cicchetti, 2013). These challenges are reflected in the phenomena of equifinality and multifinality (Cicchetti & Rogosch, 1996), namely that people can reach the same diagnosis with or without childhood adversity (equifinality), and childhood adversity can lead to multiple diagnoses (multifinality). As an example of equifinality, one study found that 70% of youth seeking treatment for social anxiety disorder had experienced maltreatment, but 30% had not (Simon et al., 2009). As an example of multifinality, exposure to childhood maltreatment can lead to multiple outcomes, such as internalizing disorders or externalizing disorders (Vachon et al., 2015).
A developmental understanding of lifetime mental health trajectories could provide insight into sources of heterogeneity and discontinuity in the pathways from childhood adversity to later psychopathology. A recent report from the longitudinal population-representative Dunedin Study revealed that 86% of individuals met criteria for at least one psychiatric diagnosis by age 45, with the majority meeting criteria for multiple diagnoses at different stages before midlife (Caspi et al., 2020). Disorder onset occurred in 59% of the sample by adolescence. The high lifetime prevalence and sequential comorbidity invites new theoretical frameworks for conceptualizing mental illness. Rather than comparing “cases” (people with mental illness) and “controls” (people without mental illness), the recent Dunedin findings suggest that our ability to disrupt the link between adversity and psychopathology comes from understanding states of mental illness, and the transitions between states of mental health and different types of mental illness within individuals across the lifespan. Stressful life events have long been recognized as among the most important triggers of these transitions from mental health to mental illness, and as sensitizers of the psychobiological response to subsequent stressors (Monroe & Harkness, 2005; Post & Weiss, 1998). However, the heterogeneity of individual responses to stressful life events remains largely unexplained. In this article, we propose a framework for parsing out this heterogeneity into stress phenotypes, defined as profiles of stress-triggered psychobiological changes that lead to high levels of clinical impairment if they do not resolve quickly, and review evidence linking specific stress phenotypes to childhood adversity. Understanding stress phenotypes will bring us closer to “precision mental health”, a person-centered approach to identifying, preventing, and treating psychopathology (Chahal et al., 2020).
Accumulating evidence supports the early-life stress sensitization hypothesis, which proposes that childhood adversity disrupts the developing biological stress response and can amplify the organism’s response to future stressors (Daskalakis et al., 2013; Levine, 2005a). To advance this hypothesis further, we contend that delineating various stress phenotypes is necessary for operationalizing these pathways. A phenotype refers to a set of observable characteristics (e.g., behaviors, cognitive performance) that mediate the effects of early-life adversity on later-life psychopathology (Bonapersona et al., 2019). Thus, we have three aims with the current paper. First, we introduce the biological and psychological complexity of the human stress response, aiming to broaden our conceptualization of the stress response towards viewing it as a whole-body biobehavioral response. Second, due to the complexity of the stress response and the widespread inter-individual and intra-individual heterogeneity in stress responses (Rab & Admon, 2020; Sapolsky, 2015), we propose the study of stress phenotypes, defined as patterns of behavior and their corresponding biological substrates elicited by stressors. Specific stress phenotypes may include reward seeking, social withdrawal, or aggression, among others. Third, we present evidence supporting the role of stress phenotypes as intermediaries between childhood adversity and later psychopathology. This work shows that stress phenotypes (i.e., behavior and corresponding biological substrates assessed under high stress such as during negative life events or laboratory stressors) are more strongly linked to both childhood adversity and psychopathology than are assessments of behavior or biology under low-stress or unstressed conditions. Under low-stress conditions, differences between people with and without experiences of childhood adversity appear subtler, and in some studies are not present unless participants are undergoing either stressful life events or an acute laboratory stress manipulation (e.g., Shalev et al., 2020; Young et al., 2019). These findings imply that childhood adversity may create specific dysregulations of the intensity and duration of the stress response. Due to our focus on development of stress phenotypes related to childhood stress exposure, we review studies that simultaneously consider concurrent stress exposure and childhood adversity, as these provide stronger evidence for specificity of developmental effects.
Before addressing these three aims, it is worth mentioning the ongoing debate in the field regarding the optimal approach for conceptualizing and defining childhood adversity (Smith & Pollak, 2020). Defining stressful experiences has been an enduring challenge since the inception of stress research (Levine, 2005b). We differentiate stressors, the events and stimuli that elicit biological changes in the organism, from stress, the organism’s psychobiological response to these provocations (Levine, 2005b). We also concur with previous arguments (Sapolsky, 2015; Smith & Pollak, 2020) that a fruitful strategy is to identify stressors in relation to their biologically-based changes, based on the activation of processes in the brain and periphery rather than socially constructed concepts of the experiences that many people find stressful (Smith & Pollak, 2020). Thus, we begin by describing the acute stress response as a biological response, with corresponding effects in affective, behavioral, cognitive, and somatic domains. We define the somatic domain as comprising psychological perceptions of bodily states, such as fatigue, pain, appetite, or insomnia. We recognize that affective, behavioral, cognitive, and somatic domains are inter-related and by necessity correlated with biological processes, although they have often been studied in isolation from each other and from the underlying biology. For example, the depression literature has often studied cognitive risk factors independently from alterations in endocrine, autonomic, and immune reactivity to stress (LeMoult, 2020). Although we review evidence from specific domains (affective, cognitive, etc.) in separate sections as they have been studied, we concur with recent calls for the field to study the reciprocal interactions among these domains in a more integrated, whole-person framework in the future (LeMoult, 2020).
The Stress Response Repertoire: Broadening Our Conceptualization of the Stress Response
The stress response is a repertoire of acute biological and behavioral responses to a “real or interpreted threat to the physiological or psychological integrity of an individual” (McEwen, 2000, p. 508). These threats can be diverse, ranging from physical demands such as pain and infections to real or perceived psychological threats such as social rejection or the fear of possible future social rejection. Stress responses depend on both the objective nature of the stressor and the individual’s appraisal of the stressor based on prior experiences and resources immediately available for coping (Lazarus & Folkman, 1984).
The acute stress response activates complex neurobiological processes that mobilize energy and organize the organism’s behavioral coping response, which may include the “fight-or-flight” response (aggression or social withdrawal), freezing, and other motivated action patterns that promote coping (McEwen & Akil, 2020). Although the hypothalamic-pituitary-adrenocortical (HPA) axis and sympathetic nervous system have been historically investigated as the canonical stress-response systems (Sapolsky, 2015), research over the past 50 years has revealed that the stress response is broader than these two systems and their end-products, cortisol and epinephrine/norepinephrine, respectively. This complex stress response has been described as a “neuro-symphony” (Joels & Baram, 2009) of changes in multiple brain circuits involving diverse neurotransmitters and neuropeptides, such as dopamine, serotonin, vasopressin, BDNF, t-PA (tissue plasminogen activator), lipocalin-2 secreted protein, and endocannabinoids among others (Joels & Baram, 2009; McEwen & Akil, 2020). In addition to these neural processes, the stress response also depends on myriad other systems and molecular players, including the immune system (Segerstrom & Miller, 2004), liver, muscles, and bones (McEwen & Akil, 2020), gut microbiota (Codagnone et al., 2019), and parasympathetic nervous system (Rab & Admon, 2020). These peripheral stress mediators have been linked to neurobehavioral outcomes, with implications for understanding the etiology of psychopathology and characterizing stress phenotypes. These diverse physiological changes orchestrate an array of stress-induced alterations in the affective, behavioral, cognitive, and somatic domains that prioritize coping with the ongoing threat over other functions (e.g., reproductive functions, digestion, growth, pursuit of long-term goals), and prepare the organism to cope with similar challenges in the future.
Considering studies focused on assessing the affective domain, a comprehensive review of human acute stress induction laboratory protocols (e.g., the Trier Social Stress Test) revealed that acute stressors increase negative affect, including anxiety, tension, irritability, shame and anger, while decreasing positive affect (Campbell & Ehlert, 2012). Acute stressors also induce alterations in reward processing, which can include anhedonia, a decrease in reward seeking, motivation and pleasure, in some individuals, as well as increased reward seeking and risk-taking in others (Stanton et al., 2019). Even brief protocols such as threat of shock in the laboratory can induce such changes (Stanton et al., 2019). Individuals who experience anhedonia after acute stressors are more prone to develop mood disorders, whereas increased reward seeking following acute stressors relates to vulnerability to substance use disorders (Stanton et al., 2019). To date, more research is needed on individual differences that predict stress-induced tendencies towards decreased or increased reward seeking, but one study revealed the role of gender, with females more prone to anhedonia and males more prone to increased reward seeking in a laboratory task conducted after an acute stressor (Lighthall et al., 2012).
Some studies have concentrated on assessing varying behavioral changes to acute stressors, with the most well studied being freezing and social behaviors (e.g., “fight-or-flight”). For instance, children exhibited more pronounced freezing behaviors in response to fear-eliciting mechanical toys if they had experienced childhood adversity in the form of orphanage rearing (Stellern et al., 2014). Experimental acute stress induction studies in humans have revealed divergent response profiles, entailing either stress-induced increases in antisocial behavior (“fight-or-flight” behaviors such as aggression, irritability, competition, or social withdrawal, social anxiety, mistrust) or increases in prosocial behaviors (“tend-and-befriend” behaviors, including altruism, caring for and willingness to cooperate with others) (Taylor, 2006; Taylor et al., 2000). Nationally-representative surveys also reveal changes in social behavior in response to acute stressors, e.g. 45% of Americans report that stress makes it more difficult to get along with family members (NPR, 2014).
In studies focusing on the cognitive domain, acute stressors have been shown to affect learning and memory. For example, memory for information related to the stressful context or negative-valence information is strengthened, whereas learning or recall of unrelated information is disrupted (Schwabe et al., 2012). Through the pervasive effects of acute stress mediators (e.g., catecholamines and glucocorticoids) on the activity of the prefrontal cortex (PFC) (Arnsten, 2015), stressors can temporarily impair core executive functions such as working memory, cognitive inhibition, and planning (Shields et al., 2016). When acute stressors become chronic, this can lead to permanent remodeling of the PFC, biasing cognition towards “reflexive” rather than “reflective” cognitive processes (Arnsten, 2015). Variable, uncontrollable stressors can also interfere with the ability to sustain attention (Eck et al., 2020).
Other studies have focused on the effects of acute stressors on the somatic domain, such as energy level, appetite, sleep and psychomotor behavior, which can be either slowed down or accelerated by stress. For example, acute stressors impact energy metabolism and regulation, and can stimulate overeating and consuming foods high in calories, fat, and sugar, presumably to store additional energy for coping with threat (Tomiyama, 2019). The majority of human studies on acute stressors and physical activity reveal an overall tendency to decrease physical activity in the aftermath of stressors and psychomotor retardation in stress-related disorders such as depression, consistent with the need to conserve energy (Stults-Kolehmainen & Sinha, 2014). However, some individuals show increased levels of activity under stress, possibly to improve their mood via physical exercise (Stults-Kolehmainen & Sinha, 2014). Animal models document decreased motor activity and increased fatigue after stressors, and their mediation by stress-induced production of proinflammatory cytokines (Dantzer et al., 2008). Human research similarly finds that stressful events induce sleep disruptions, which can produce fatigue and metabolic changes (Akerstedt et al., 2007).
Although studies have revealed this broad range of behavioral and biological changes typically elicited by acute stressors, there is considerable variability across individuals and across stressors in the magnitude of the effects and the specific constellation of effects experienced (Rab & Admon, 2020). This variability may be useful for interrogating the pathways from childhood adversity to psychopathology.
Stress Phenotypes: Profiles of Stress Responses
Research on the effects of acute stressors on human biology and behavior suggests two major conclusions. First, many clinical symptoms (e.g., anhedonia, psychomotor slowing, cognitive deficits) of various psychiatric diagnoses are commonly experienced as transient components of the stress response in healthy participants exposed to experimental laboratory stressors. What remains unknown are the mechanisms that facilitate the persistence of these symptoms over time, as observed in clinical disorders. Second, there is wide heterogeneity of individual stress response repertoires, which is likely a product of multiple interacting influences, including genetic variation, developmental context and history, and external intervention such as clinical treatment (McEwen & Akil, 2020; Rab & Admon, 2020).
It is plausible then that the heterogeneity seen in stress-response profiles may be useful for predicting future psychopathology. For example, a meta-analysis of studies with healthy humans revealed acute stress laboratory induction protocols can induce executive function deficits (Shields et al., 2016), and greater executive function deficits following stressor exposure are more strongly predictive of depression than is baseline, pre-stressor executive function task performance (Quinn & Joormann, 2015a, 2015b). Furthermore, as reviewed above, acute stressors alter reward seeking and social behavior, but individuals can show completely opposite profiles of stress-related alterations in these domains, such that decreases and increases in reward seeking under stress predict different forms of psychopathology (Stults-Kolehmainen & Sinha, 2014). These findings highlight a need to characterize more fully the mechanistic underpinnings for these divergent tendencies.
The prior literature on acute stress effects has thus far focused predominantly on discrete aspects of biological and behavioral profiles (e.g., effects of stress either on affect or on cognition). More research is needed to reveal how specific effects of stress on affect, behavior, cognition, and somatic states are inter-related with each other. We propose a framework (Figure 1) to guide research focused on conceptualizing these effects as components of stress response profiles that are integrative across domains and gain a richer understanding of the biological underpinnings of these stress profiles.
Figure 1.
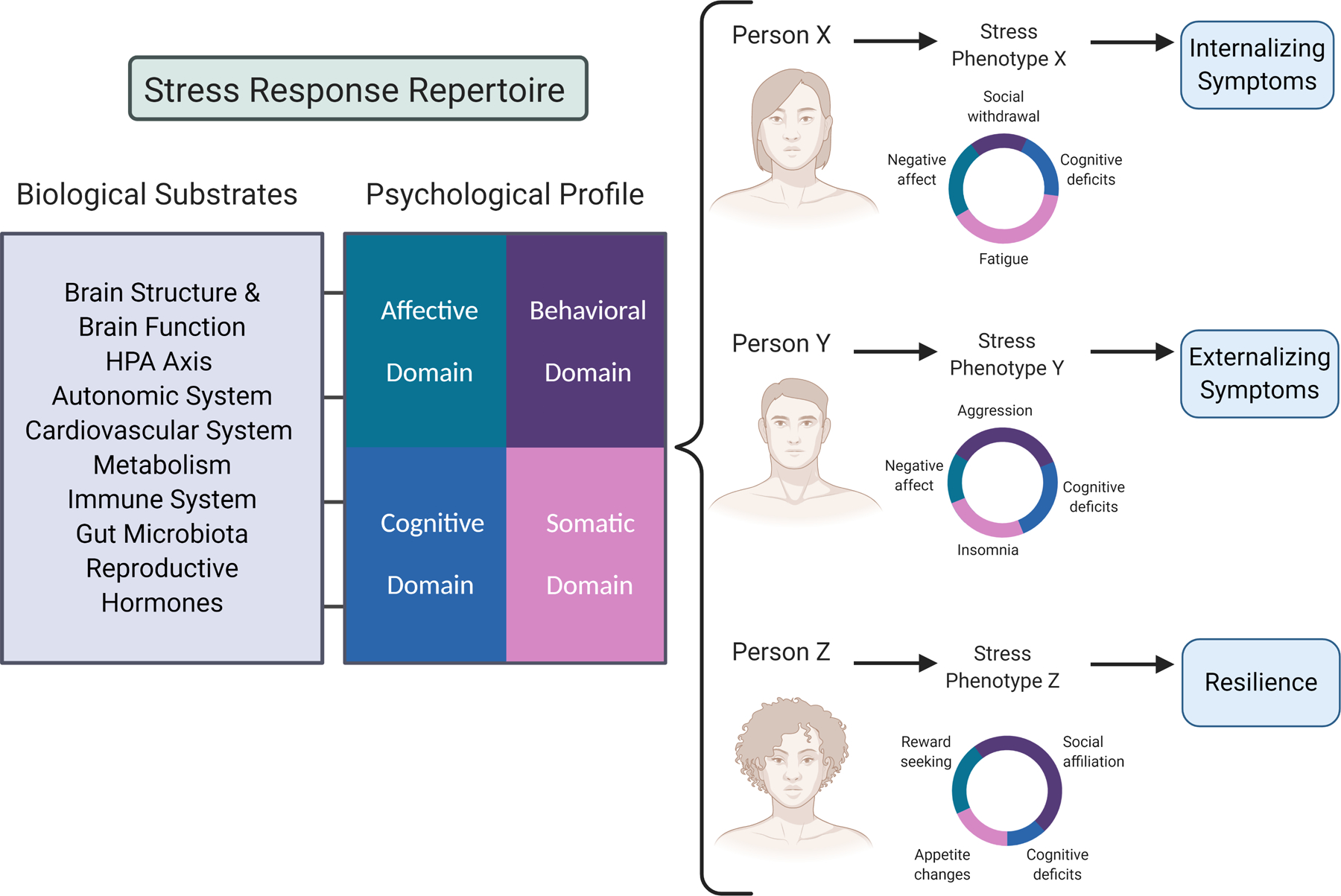
Framework for studying psychobiological stress phenotypes. Future empirical research would document the inter-related affective, behavioral, cognitive, and somatic changes in response to acute stress for each individual using large samples, and aim to uncover the biological substrates for these effects. We hypothesize variability in individual stress phenotypes (i.e., profiles) of stress responses. Acknowledging that there are multiple elements within each domain, this figure presents a simplified representation for explanatory purposes. As hypothetical examples, Person X might show a high increase in negative affect, social withdrawal, cognitive deficits, and somatic symptoms such as fatigue post-stress, which may predispose them to developing elevated internalizing symptoms over time. Person Y might show stress-related increases in negative affect, aggressive behaviors, cognitive deficits, and insomnia, a profile which over time may become crystallized into elevated externalizing symptoms. Finally, Person Z may show a large change in the behavioral domain, specifically an increase in reward seeking and social-affiliative behavior, which would buffer their endocrine stress response and attenuate the negative affective, cognitive, and somatic effects of the stressor, leading to a profile of resilience to psychopathology. Person-centered analytic methods could identify these and other profiles that characterize biobehavioral subgroups within a population that are prone toward distinct mental health trajectories. This would be particularly fruitful within populations exposed to childhood adversity, who may show different or accentuated stress-response profiles within and across affective, behavioral, cognitive, and somatic domains. Figure created with BioRender.com.
Using this framework, we envision that researchers would first use meta-analyses, machine learning, and other data science techniques to catalogue available human research on affective, cognitive, behavioral, and somatic effects of acute stress, as well as biological alterations corresponding to these effects, in order to compile a complete human stress response repertoire. Then, new empirical research using a range of different types of naturalistic or laboratory stressors would be used to examine individual stress phenotypes across these multiple domains. The goal would be to assess the magnitude of change in each of these domains from pre- to post-stress for each individual (Figure 1), and assess which affective, behavioral, cognitive and somatic components cluster together. This would be a necessary step toward the goal of uncovering biological substrates that distinguish the profiles, and these substrates may predict to psychopathology either independently or in combination with the psychological profiles. For example, the constellation of stress-induced “sickness behaviors,” including behaviors such as social withdrawal and somatic states such as fatigue, psychomotor slowing, and increased slow-wave sleep, has been linked to increased production of proinflammatory cytokines and development of depressive-like behavior in animal models (Dantzer et al., 2008). Post-stress increases and decreases in oxytocin have been linked to affiliative and aggressive behaviors, respectively, in primate models (Witczak et al., 2018). Just as animal models indicate that specific biological mechanisms distinguish acute stress responses, identifying acute stress phenotypes in humans could reveal discernable biological substrates. Furthermore, this approach would allow us to parse variability of stress phenotypes into trait-level components that may be characteristic of individuals versus state-level components that may be closely related to context and stressor type. Stress responses that are typically or commonly evidenced across multiple provocations are more likely to be informative about consistent person-centered biobehavioral processes that convey greater or lesser risk for psychopathology.
To support the argument that characterizing stress phenotypes may advance our understanding of pathways from childhood adversity to psychopathology, we review evidence showing that stress phenotypes are more strongly linked to childhood adversity and psychopathology than psychobiological states assessed under low stress.
Stress Phenotypes as Intermediaries between Childhood Adversity and Psychopathology
Intriguingly, accumulating evidence shows that assessing participants under stressful circumstances – either naturalistic or laboratory – may increase predictive power from childhood adversity to clinical outcomes or to purported mediators in the pathway to clinical outcomes. Childhood adversity is associated with alterations in affective, behavioral, cognitive and somatic domains. Some studies have uncovered clues about the biological substrates for these effects and examined biobehavioral, multi-domain stress phenotypes, including assessments of how biology and behavior are inter-related. We organize existing evidence into affective, behavioral, cognitive, and somatic phenotypes, concluding with a section on biological substrates and multi-domain phenotypes, as we recognize that the most promising future avenue for the field is to move beyond individual domains to study multi-domain phenotypes in relation to biological substrates.
Affective Domain
In the affective domain, several studies have documented stronger increases in negative affect in association with concurrent stress among individuals with childhood adversity exposure compared to those without this exposure (Cristobal-Narvaez et al., 2016; Glaser et al., 2006; Weltz et al., 2016; Yaroslavsky et al., 2020). Using ecological momentary assessment, one such study collected subjective ratings of both event-related stress levels and negative affect 10 times per day on six consecutive days (Glaser et al., 2006). Among individuals with a history of childhood trauma, higher stress ratings were associated with higher concurrent ratings of negative affect, though this association was not observed among those without a history of trauma. Childhood trauma exposure has also been linked to greater subjective appraisal of daily stressful events as stressful (LoPilato et al., 2020) and greater subjective appraisal of an acute laboratory stressor as stressful by adults (Zhong et al., 2020). In adolescents with a history of childhood adversity, greater recent life stress has been associated with more internalizing symptoms (Ruttle et al., 2014) and greater prevalence of suicidal ideation (A. B. Miller et al., 2017). Further, studies in the affective domain have investigated how acute stress exposure influences processing of emotional information among adolescents with a history of adversity. For instance, experiencing more lifetime stressors increased the effect of an acute social stressor, causing 11–15 year-old youths to rely more on facial affect information and less on other available information in an emotion recognition task (Smith et al., 2020).
Behavioral Domain
Evidence suggests that childhood adversity strengthens the association between recent stressor exposure and behavior problems. Some striking evidence comes from a randomized controlled trial of foster care placement of children reared in Romanian orphanages. Among adolescents reared in this institutional setting, a greater number of recent life stressors over the previous year was associated with more externalizing behavior problems (Wade et al., 2019). This association was not observed, however, among adolescents who were randomly assigned to foster care placement early in childhood, or among an age-matched control group of adolescents never exposed to institutionalized rearing (Wade et al., 2019). Experiencing life stressors over the previous year has also been associated with increased odds of recent intimate partner violence among adults with a history of childhood adversity, but again this link was not observed among individuals without a history of childhood adversity (Roberts et al., 2011). In another study, greater levels of uncontrollable life stressors over the previous year were associated with greater alcohol consumption over the same year period, but only among women with a history of childhood maltreatment (Young-Wolff et al., 2012).
Cognitive Domain
Childhood adversity has been associated with reduced performance in multiple domains of cognitive functioning, including attention, executive function, memory, as well as in global measures such as IQ or academic achievement (De Bellis et al., 2009; De Bellis et al., 2013; Gould et al., 2012). However, a smaller but growing literature has documented that children experiencing adversity exhibit strengths in some facets of attention, perception, learning, memory, and problem solving (Ellis et al., 2017). Although these inconsistencies may relate to the types of tasks and environmental contexts involved in these assessments, it is also possible that these heterogeneous outcomes are partially due to variability in the level of life stress exposure that children were experiencing at the time of testing. In support of this hypothesis, one study showed that cognitive and behavioral strategies used for emotion regulation mediated the association between a history of childhood sexual abuse and depressive symptoms, but this pathway was only significant among individuals who reported high levels of recent stress over the previous month (Yaroslavsky et al., 2020). However, more research is needed to determine the extent to which concurrent stressors reveal cognitive deficits versus strengths among those previously exposed to childhood adversity. Some neuroimaging studies have also revealed differences in cognitive processing under stress for those exposed to childhood adversity, and we review these below in the section on biological substrates.
Somatic Domain
In the somatic domain, daily diary studies have linked greater recent stress levels with poorer sleep (Hanson & Chen, 2010) and more somatic symptoms such as pain and fatigue (Thakkar & McCanne, 2000). However, in each of these studies the relations were only significant among individuals with a history of childhood adversity (Hanson & Chen, 2010; Thakkar & McCanne, 2000). Another recent study of American Indian adults revealed that childhood adversity was associated with greater increases in psychological stress one month after the onset of the COVID-19 pandemic, which mediated greater declines in sleep quality from baseline to one month post-pandemic onset (John-Henderson, 2020).
In sum, there is substantial evidence that the combination of childhood adversity and current heightened stress reveals important individual differences in affect, behavior, cognition, and somatic functioning. However, substantial gaps still exist. Almost all of the above findings focused on naturalistic stressors. While there are benefits to using ecologically valid measures of stressors, stronger causal evidence may come from measuring changes in each of these domains after random assignment to an acute laboratory stressor. More research is needed to link these stress phenotypes to their biological substrates, although some studies on multi-domain biobehavioral phenotypes are underway.
Biological Substrates and Multi-domain Profiles
Many existing models of psychopathology aim to understand the specific dimensions of childhood adversity that convey risk for psychopathology and the mechanistic pathways for how they elevate risk. This literature has indeed revealed a number of biological mediators of the effect of childhood adversity on risk for psychopathology. We review evidence on hormonal, immune, and neural mediators, as these mediators have been assessed most frequently in studies that simultaneously consider the joint roles of childhood adversity and recent stressors.
For example, in a 37-year prospective longitudinal study, childhood adversity was linked to flatter cortisol slopes in adulthood at age 37 if participants were experiencing relatively high levels of concurrent stress, but not otherwise (Young et al., 2019). Childhood adversity has also been associated with greater cortisol reactivity to an acute social stressor, whereas no relation was observed between childhood adversity and baseline cortisol levels (Shalev et al., 2020). In another study on inflammatory markers, exposure to parental harshness during childhood interacted with recent stressors to predict greater production of interleukin-6 (IL-6) in response to an in vitro immune challenge among adolescent females, but parental harshness did not show direct associations with circulating levels of IL-6 outside of the immune challenge and in the absence of recent stressors (G. E. Miller & Chen, 2010). Elevated circulating IL-6 levels have been observed among adults who experienced high stress on the day preceding testing and who had a history of childhood maltreatment, whereas this relation was not observed among those without a history of maltreatment (Gouin et al., 2012).
Neuroimaging evidence has shown that measuring the brain’s function under stress may lead to stronger links with childhood adversity than measuring brain function under non-stressful conditions. For example, Golde et al. (2019) examined neural activity under acute stress in a sample of women with severe childhood trauma and compared them to women with no history of trauma. Women with childhood trauma exhibited blunted inferior frontal gyrus (IFG) activity during an emotional response inhibition task after acute stress manipulation, relative to the women with no trauma history, whereas trauma history was not associated with neural activity to the task administered prior to the stress manipulation. These results suggest that blunted activity post-stressor in the IFG, which is involved in cognitive control and emotion regulation, may be a stress phenotype associated with childhood trauma. Other research has shown that childhood maltreatment predicts increased amygdala-hippocampus connectivity under acute stress (Fan et al., 2015) and that childhood poverty predicts increased insula activation during emotion processing under acute stress (Liberzon et al., 2015). Thus, acute stress may be required to reveal some of these stress phenotypes involving direct measures of brain function associated with childhood adversity. However, much of this work was conducted in adults with no current or past psychiatric disorders (Fan et al., 2015; Golde et al., 2019; Liberzon et al., 2015; Seo et al., 2014), making it unclear which patterns of activity may indicate future risk for psychopathology or markers of resilience (Guyer, 2020).
Research in clinical samples has also shown that brain function under stress may be more strongly linked to psychopathology than brain function in non-stress conditions. For example, Kumar et al. (2015) examined brain stress phenotypes in 12 adults with major depression and 10 healthy controls. Participants underwent an MRI scan and performed a monetary reward motivation task under two conditions, baseline (no-stress) and acute stress. Acute stress was induced by giving participants negative feedback and imposing a monetary penalty for slow responses on certain trials. During the acute stress manipulation, participants showed increased activity to reward in the medial PFC (mPFC), a region that communicates with the ventral striatum to process information about reward. Participants with major depression who reported greater perceived life stress severity evidenced larger increases in activity in the mPFC during acute stress relative to the no-stress condition. In contrast, there were not significant effects of acute stress on mPFC activity in the healthy, non-depressed control group. Increased mPFC activity during acute stress may underlie some of the symptoms and behaviors characteristic of depression, such as learned helplessness and anhedonia (Kumar et al., 2015).
There is also substantial evidence that alterations in reward processing and reward-sensitivity mediate the relation between childhood adversity and psychopathology (for review, see Herzberg & Gunnar, 2020). Interestingly, studies suggest this indirect effect may be stronger in the presence of current or recent stressor exposure. For example, increased reward-related functional connectivity between the ventral striatum and mPFC during a monetary reward card-guessing game mediated the relation between retrospective reports of childhood maltreatment and depressive symptoms (Hanson et al., 2018). Importantly, this indirect effect was only significant for individuals who reported greater levels of recent stressful life events (Hanson et al., 2018). Another study observed links between blunted reward sensitivity at age 9 and depression at age 12, but this association was only significant in the presence of high recent life stress at age 12 (Goldstein et al., 2020).
In sum, neuroimaging research has begun to show that measuring brain function under acute stress or recent life stress may reveal stress phenotypes linked to childhood adversity and psychopathology that would not be observed through measures of neural activity under lower stress conditions. Further research is needed to examine whether these brain stress phenotypes mediate the association between childhood adversity and the development of psychopathology.
Conclusions
The evidence reviewed here suggests that childhood adversity may create a specific vulnerability in regulating the stress response and recovering from it. Furthermore, we reviewed evidence that symptoms such as anhedonia, reward seeking, executive function deficits, and aggression can be elicited in transient forms in healthy individuals using acute laboratory stress inductions. Together, these lines of evidence suggest some forms of psychopathology may in fact be non-resolving high-stress states, which prevent ongoing adaptation to the environment. More research is needed to usher a mechanistic understanding of these non-resolving high-stress states, and test whether they occur due to factors endogenous to the individual (e.g., lower threshold for activation of the stress response, dysregulation of negative feedback mechanisms that typically terminate the stress response), or due to unremitting exogenous stressors, or some combination of both. Understanding stress phenotypes, the specific and inter-related affective, behavioral, cognitive, and somatic profiles of changes in the aftermath of stressors, as well as their biological substrates, may lead to greater specificity and accuracy of prediction of future mental health symptoms. The specific constellation of changes in child and adolescent behavior under mild stress may function as an individualized omen of and window into future states of psychopathology that may develop under high stress. The vision is that a highly tailored assessment of individuals’ biobehavioral stress profiles at various points in time will pave the way for more effective therapies across a range of psychopathologies.
Acknowledgements:
This manuscript was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD0938898. The views expressed do not necessarily represent those of the US government.
References
- Akerstedt T, Kecklund G, & Gillberg M (2007). Sleep and sleepiness in relation to stress and displaced work hours. Physiology & Behavior, 92(1–2), 250–255. doi: 10.1016/j.physbeh.2007.05.044 [DOI] [PubMed] [Google Scholar]
- Arnsten AF (2015). Stress weakens prefrontal networks: Molecular insults to higher cognition. Nat Neurosci, 18(10), 1376–1385. doi: 10.1038/nn.4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonapersona V, Kentrop J, Van Lissa CJ, van der Veen R, Joels M, & Sarabdjitsingh RA (2019). The behavioral phenotype of early life adversity: A 3-level meta-analysis of rodent studies. Neuroscience & Biobehavioral Reviews, 102, 299–307. doi: 10.1016/j.neubiorev.2019.04.021 [DOI] [PubMed] [Google Scholar]
- Campbell J, & Ehlert U (2012). Acute psychosocial stress: Does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology, 37(8), 1111–1134. doi: 10.1016/j.psyneuen.2011.12.010 [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Ambler A, Danese A, Elliott ML, Hariri A, … Moffitt TE (2020). Longitudinal assessment of mental health disorders and comorbidities across 4 decades among participants in the Dunedin Birth Cohort Study. Jama Network Open, 3(4), e203221. doi: 10.1001/jamanetworkopen.2020.3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal R, Gotlib IH, & Guyer AE (2020). Brain network connectivity and the heterogeneity of depression in adolescence - A precision mental health perspective. Journal of Child Psychology and Psychiatry. doi: 10.1111/jcpp.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, & Rogosch FA (1996). Equifinality and multifinality in developmental psychopathology. Development and Psychopathology, 8(4), 597–600. doi: 10.1017/S0954579400007318 [DOI] [Google Scholar]
- Cicchetti D, Rogosch FA, & Toth SL (2006). Fostering secure attachment in infants in maltreating families through preventive interventions. Development and Psychopathology, 18(3), 623–649. doi: 10.1017/S0954579406060329 [DOI] [PubMed] [Google Scholar]
- Codagnone MG, Spichak S, O’Mahony SM, O’Leary OF, Clarke G, Stanton C, … Cryan JF (2019). Programming bugs: Microbiota and the developmental origins of brain health and disease. Biological Psychiatry, 85(2), 150–163. doi: 10.1016/j.biopsych.2018.06.014 [DOI] [PubMed] [Google Scholar]
- Costello EJ, Erkanli A, Copeland W, & Angold A (2010). Association of family income supplements in adolescence with development of psychiatric and substance use disorders in adulthood among an American Indian Population. JAMA, 303(19), 1954–1960. doi: 10.1001/jama.2010.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristobal-Narvaez P, Sheinbaum T, Ballespi S, Mitjavila M, Myin-Germeys I, Kwapil TR, & Barrantes-Vidal N (2016). Impact of adverse childhood experiences on psychotic-like symptoms and stress reactivity in daily life in nonclinical young adults. PLoS One, 11(4). doi:ARTNe0153557 10.1371/journal.pone.0153557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, & Kelley KW (2008). From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience, 9(1), 46–56. doi: 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, & de Kloet ER (2013). The three-hit concept of vulnerability and resilience: Toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology, 38(9), 1858–1873. doi: 10.1016/j.psyneuen.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Hooper SR, Spratt EG, & Woolley DP (2009). Neuropsychological findings in childhood neglect and their relationships to pediatric PTSD. Journal of the International Neuropsychological Society, 15(6), 868–878. doi: 10.1017/S1355617709990464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Woolley DP, & Hooper SR (2013). Neuropsychological findings in pediatric maltreatment: Relationship of PTSD, dissociative symptoms, and abuse/neglect indices to neurocognitive outcomes. Child Maltreatment, 18(3), 171–183. doi: 10.1177/1077559513497420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M, Roben CKP, Caron EB, Hoye J, & Bernard K (2018). Attachment and Biobehavioral Catch-up: An evidence-based intervention for vulnerable infants and their families. Psychotherapy Research, 28(1), 18–29. doi: 10.1080/10503307.2016.1229873 [DOI] [PubMed] [Google Scholar]
- Eck SR, Xu SJ, Telenson A, Duggan MR, Cole R, Wicks B, … Bangasser DA (2020). Stress regulation of sustained attention and the cholinergic attention system. Biological Psychiatry, 88(7), 566–575. doi: 10.1016/j.biopsych.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Bianchi J, Griskevicius V, & Frankenhuis WE (2017). Beyond risk and protective factors: An adaptation-based approach to resilience. Perspectives on Psychological Science, 12(4), 561–587. doi: 10.1177/1745691617693054 [DOI] [PubMed] [Google Scholar]
- Fan Y, Pestke K, Feeser M, Aust S, Pruessner JC, Boker H, … Grimm S (2015). Amygdala-hippocampal connectivity changes during acute psychosocial stress: Joint effect of early life stress and oxytocin. Neuropsychopharmacology, 40, 2736–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser JP, van Os J, Portegijs PJM, & Myin-Germeys I (2006). Childhood trauma and emotional reactivity to daily life stress in adult frequent attenders of general practitioners. Journal of Psychosomatic Research, 61(2), 229–236. doi: 10.1016/j.jpsychores.2006.04.014 [DOI] [PubMed] [Google Scholar]
- Golde S, Wingenfeld K, Riepenhausen A, Schroter N, Fleischer J, Prussner J, … Otte C (2019). Healthy women with severe early life trauma show altered neural facilitation of emotion inhibition under acute stress. Psychological Medicine, 1–10. [DOI] [PMC free article] [PubMed]
- Goldstein BL, Kessel EM, Kujawa A, Finsaas MC, Davila J, Hajcak G, & Klein DN (2020). Stressful life events moderate the effect of neural reward responsiveness in childhood on depressive symptoms in adolescence. Psychological Medicine, 50(9), 1548–1555. doi: 10.1017/S0033291719001557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin JP, Glaser R, Malarkey WB, Beversdorf D, & Kiecolt-Glaser JK (2012). Childhood abuse and inflammatory responses to daily stressors. Annals of Behavioral Medicine, 44(2), 287–292. doi: 10.1007/s12160-012-9386-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould F, Clarke J, Heim C, Harvey PD, Majer M, & Nemeroff CB (2012). The effects of child abuse and neglect on cognitive functioning in adulthood. Journal of Psychiatric Research, 46(4), 500–506. doi: 10.1016/j.jpsychires.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE (2020). Adolescent psychopathology: The role of brain-based diatheses, sensitivities, and susceptibilities. Child Development Perspectives. doi: 10.1111/cdep.12365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Knodt AR, Brigidi BD, & Hariri AR (2018). Heightened connectivity between the ventral striatum and medial prefrontal cortex as a biomarker for stress-related psychopathology: Understanding interactive effects of early and more recent stress. Psychological Medicine, 48(11), 1835–1843. doi: 10.1017/S0033291717003348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MD, & Chen E (2010). Daily stress, cortisol, and sleep: The moderating role of childhood psychosocial environments. Health Psychology, 29(4), 394–402. [DOI] [PubMed] [Google Scholar]
- Herzberg MP, & Gunnar MR (2020). Early life stress and brain function: Activity and connectivity associated with processing emotion and reward. Neuroimage, 209. doi:ARTN 116493 10.1016/j.neuroimage.2019.116493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, & Baram TZ (2009). The neuro-symphony of stress. Nature Reviews Neuroscience, 10(6), 459–466. doi: 10.1038/nrn2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John-Henderson NA (2020). Childhood trauma as a predictor of changes in sleep quality in American Indian adults during the COVID-19 pandemic. Sleep Health. doi: 10.1016/j.sleh.2020.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, … Williams DR (2010). Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. British Journal of Psychiatry, 197(5), 378–385. doi: 10.1192/bjp.bp.110.080499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Slavich GM, Berghorst LH, Treadway MT, Brooks NH, Dutra SJ, … Pizzagalli DA (2015). Perceived life stress exposure modulates reward-related medial prefrontal cortex responses to acute stress in depression. Journal of Affective Disorders, 180, 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus RS, & Folkman S (1984). Stress, appraisal, and coping. New York, NY: Springer. [Google Scholar]
- LeMoult J (2020). From stress to depression: Bringing together cognitive and biological science. Current Directions in Psychological Science, 29(6), 592–598. 10.1177/0963721420964039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S (2005a). Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology, 30(10), 939–946. doi: 10.1016/j.psyneuen.2005.03.013 [DOI] [PubMed] [Google Scholar]
- Levine S (2005b). Stress: An historical perspective. In Steckler T, Kalin NH, & Reul JM (Eds.), Handbook of Stress and the Brain (pp. 3–23).
- Liberzon I, Ma ST, Okada G, Ho SS, Swain JE, & Evans GW (2015). Childhood poverty and recruitment of adult emotion regulatory neurocircuitry. Social Cognitive and Affective Neuroscience, 10, 1596–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthall NR, Sakaki M, Vasunilashorn S, Nga L, Somayajula S, Chen EY, … Mather M (2012). Gender differences in reward-related decision processing under stress. Social Cognitive and Affective Neuroscience, 7(4), 476–484. doi: 10.1093/scan/nsr026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPilato AM, Addington J, Bearden CE, Cadenhead KS, Cannon TD, Cornblatt BA, … Walker EF (2020). Stress perception following childhood adversity: Unique associations with adversity type and sex. Development and Psychopathology, 32(1), 343–356. doi:PiiS0954579419000130 10.1017/S0954579419000130 [DOI] [PubMed] [Google Scholar]
- Masten AS, & Narayan AJ (2012). Child development in the context of disaster, war, and terrorism: Pathways of risk and resilience. Annual Review of Psychology, 63, 227–257. doi: 10.1146/annurev-psych-120710-100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Akil H (2020). Revisiting the stress concept: Implications for affective disorders. Journal of Neuroscience, 40(1), 12–21. doi: 10.1523/Jneurosci.0733-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AB, Eisenlohr-Moul T, Giletta M, Hastings PD, Rudolph KD, Nock MK, & Prinstein MJ (2017). A within-person approach to risk for suicidal ideation and suicidal Behavior. Journal of Consulting and Clinical Psychology, 85(7), 712–722. doi: 10.1037/ccp0000210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, & Chen E (2010). Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science, 21(6), 848–856. doi: 10.1177/0956797610370161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, & Harkness KL (2005). Life stress, the “Kindling” hypothesis, and the recurrence of depression: Considerations from a life stress perspective. Psychological Review, 112(2), 417–445. doi: 10.1037/0033-295x.112.2.417 [DOI] [PubMed] [Google Scholar]
- Morris PA, Aber JL, Wolf S, & Berg J (2017). Impacts of family rewards on adolescents’ mental health and problem behavior: Understanding the full range of effects of a conditional cash transfer program. Prevention Science, 18(3), 326–336. doi: 10.1007/s11121-017-0748-6 [DOI] [PubMed] [Google Scholar]
- NPR. (2014). Burden of Stress in America. Retrieved from https://media.npr.org/documents/2014/july/npr_rwfj_harvard_stress_poll.pdf
- Post RM, & Weiss SRB (1998). Sensitization and kindling phenomena in mood, anxiety, and obsessive-compulsive disorders: The role of serotonergic mechanisms in illness progression. Biological Psychiatry, 44(3), 193–206. doi:Doi 10.1016/S0006-3223(98)00144-9 [DOI] [PubMed] [Google Scholar]
- Quinn ME, & Joormann J (2015a). Control when it counts: Change in executive control under stress predicts depression symptoms. Emotion, 15(4), 522–530. doi: 10.1037/emo0000089 [DOI] [PubMed] [Google Scholar]
- Quinn ME, & Joormann J (2015b). Stress-induced changes in executive control are associated with depression symptoms: Examining the role of rumination. Clinical Psychological Science, 3(4) 628–636. doi: 10.1177/2167702614563930 [DOI] [Google Scholar]
- Rab SL, & Admon R (2020). Parsing inter- and intra-individual variability in key nervous system mechanisms of stress responsivity and across functional domains. Neuroscience & Biobehavioral Reviews. doi: 10.1016/j.neubiorev.2020.09.007 [DOI] [PubMed] [Google Scholar]
- Roberts AL, McLaughlin KA, Conron KJ, & Koenen KC (2011). Adulthood stressors, history of childhood adversity, and risk of perpetration of intimate partner violence. American Journal of Preventive Medicine, 40(2), 128–138. doi: 10.1016/j.amepre.2010.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttle PL, Armstrong JM, Klein MH, & Essex MJ (2014). Adolescent internalizing symptoms and negative life events: The sensitizing effects of earlier life stress and cortisol. Development and Psychopathology, 26(4), 1411–1422. doi: 10.1017/S0954579414001114 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM (2015). Stress and the brain: Individual variability and the inverted-U. Nature Neuroscience, 18(10), 1344–1346. doi: 10.1038/nn.4109 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Joels M, Roozendaal B, Wolf OT, & Oitzl MS (2012). Stress effects on memory: An update and integration. Neurosci Biobehav Rev, 36(7), 1740–1749. doi: 10.1016/j.neubiorev.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, & Miller GE (2004). Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin, 130(4), 601–630. doi: 10.1037/0033-2909.130.4.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Tsou KA, Ansell EB, Potenza MN, & Sinha R (2014). Cumulative adversity sensitizes neural response to acute stress: Association with health symptoms. Neuropsychopharmacology, 39, 670–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Hastings WJ, Etzel L, Israel S, Russell MA, Hendrick KA, … Siegel SR (2020). Investigating the impact of early-life adversity on physiological, immune, and gene expression responses to acute stress: A pilot feasibility study. PLoS One, 15(4). doi:ARTN e0221310 10.1371/journal.pone.0221310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Sazma MA, & Yonelinas AP (2016). The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neuroscience and Biobehavioral Reviews, 68, 651–668. doi: 10.1016/j.neubiorev.2016.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NM, Herlands NN, Marks EH, Mancini C, Letamendi A, Li ZH, … Stein MB (2009). Childhood maltreatment linked to greater symptom severity and poorer quality of life and function in social anxiety disorder. Depression and Anxiety, 26(11), 1027–1032. doi: 10.1002/da.20604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Leitzke BT, & Pollak SD (2020). Youths’ processing of emotion information: Responses to chronic and video-based laboratory stress. Psychoneuroendocrinology, 122, 104873. doi: 10.1016/j.psyneuen.2020.104873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, & Pollak SD (2020). Rethinking concepts and categories for understanding the neurodevelopmental effects of childhood adversity. Perspectives on Psychological Science. doi:1745691620920725 10.1177/1745691620920725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton CH, Holmes AJ, Chang SWC, & Joormann J (2019). From stress to anhedonia: Molecular processes through functional circuits. Trends in Neurosciences, 42(1), 23–42. doi: 10.1016/j.tins.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellern S, Esposito E, Mliner S, Pears K, & Gunnar M (2014). Increased freezing and decreased positive affect in postinstitutionalized children. Journal of Child Psychology and Psychiatry, 55(1), 88–95. doi: 10.1111/jcpp.12123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stults-Kolehmainen MA, & Sinha R (2014). The effects of stress on physical activity and exercise. Sports Medicine, 44(1), 81–121. doi: 10.1007/s40279-013-0090-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE (2006). Tend and befriend: Biobehavioral bases of affiliation under stress. Current Directions in Psychological Science, 15(6), 273–277. doi: 10.1111/j.1467-8721.2006.00451.x [DOI] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, & Updegraff JA (2000). Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review, 107(3), 411–429. doi: 10.1037/0033-295x.107.3.411 [DOI] [PubMed] [Google Scholar]
- Thakkar RR, & McCanne TR (2000). The effects of daily stressors on physical health in women with and without a childhood history of sexual abuse. Child Abuse & Neglect, 24(2), 209–221. doi: 10.1016/S0145-2134(99)00129-5 [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ (2019). Stress and obesity. Annual Review of Psychology, 70, 703–718. doi: 10.1146/annurev-psych-010418-102936 [DOI] [PubMed] [Google Scholar]
- Vachon DD, Krueger RF, Rogosch FA, & Cicchetti D (2015). Assessment of the harmful psychiatric and behavioral effects of different forms of child maltreatment. JAMA Psychiatry, 72(11), 1135–1142. doi: 10.1001/jamapsychiatry.2015.1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade M, Zeanah CH, Fox NA, Tibu F, Ciolan LE, & Nelson CA (2019). Stress sensitization among severely neglected children and protection by social enrichment. Nature Communications, 10. doi:ARTN 5771 10.1038/s41467-019-13622-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltz SM, Armeli S, Ford JD, & Tennen H (2016). A daily process examination of the relationship between childhood trauma and stress-reactivity. Child Abuse & Neglect, 60, 1–9. doi: 10.1016/j.chiabu.2016.08.005 [DOI] [PubMed] [Google Scholar]
- Witczak LR, Ferrer E, & Bales KL (2018). Effects of aggressive temperament on endogenous oxytocin levels in adult titi monkeys. American Journal of Primatology, 80(10). doi:ARTNe22907 10.1002/ajp.22907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Bush AH, & France CM (2020). Emotion regulation deficits mediate childhood sexual abuse effects on stress sensitization and depression outcomes. Development and Psychopathology, 1–14. doi: 10.1017/S095457942000098X [DOI] [PubMed] [Google Scholar]
- Young-Wolff KC, Kendler KS, & Prescott CA (2012). Interactive effects of childhood maltreatment and recent stressful life events on alcohol consumption in adulthood. Journal of Studies on Alcohol and Drugs, 73(4), 559–569. doi: 10.15288/jsad.2012.73.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ES, Farrell AK, Carlson EA, Englund MM, Miller GE, Gunnar MR, … Simpson JA (2019). The dual impact of early and concurrent life stress on adults’ diurnal cortisol patterns: A prospective study. Psychological Science, 30(5), 739–747. doi: 10.1177/0956797619833664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Ming QS, Dong DF, Sun XQ, Cheng C, Xiong G, … Yao SQ (2020). Childhood maltreatment experience influences neural response to psychosocial stress in adults: An fMRI Study. Frontiers in Psychology, 10. doi:ARTN2961 10.3389/fpsyg.2019.02961 [DOI] [PMC free article] [PubMed] [Google Scholar]


