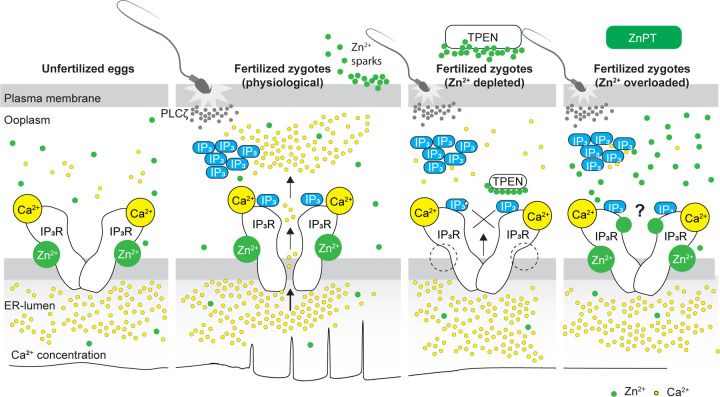
Figure 7. Schematic of proposed regulation of IP3R1 function by Zn2+ in eggs and fertilized zygotes.
In MII eggs, left panel, IP3R1s are in a Ca2+-release permissive state with optimal levels of cytoplasmic Ca2+ and Zn2+ and maximum ER content, but Ca2+ is maintained at resting levels by the combined actions of pumps, ER Ca2+ leak, and reduced influx. Once fertilization takes place, left center panel, robust IP3 production induced by the sperm-borne PLCζ leads to Ca2+ release through ligand-induced gating of IP3R1. Continuous IP3 production and refilling of the stores via Ca2+ influx ensure the persistence of the oscillations. Zn2+ release occurs in association with first few Ca2+ rises and cortical granule exocytosis, Zn2+ sparks, lowering Zn2+ levels but not sufficiently to inhibit IP3R1 function. Zn2+ deficiency caused by TPEN or other permeable Zn2+ chelators, right center panel, dose-dependently impairs IP3R1 function and limits Ca2+ release. We propose this is accomplished by stripping the Zn2+ bound to the residues of the zinc-finger motif in the LNK domain of IP3R1 that prevents the allosteric modulation of the gating process induced by IP3 or other agonists. We propose that excess Zn2+, right panel, also inhibits IP3R1-mediate Ca2+ release, possibly by non-specific binding of thiol groups present in cysteine residues throughout the receptor (denoted by a ?). We submit that optimal Ca2+ oscillations in mouse eggs unfold in the presence of a permissive range of Zn2+ concentration.