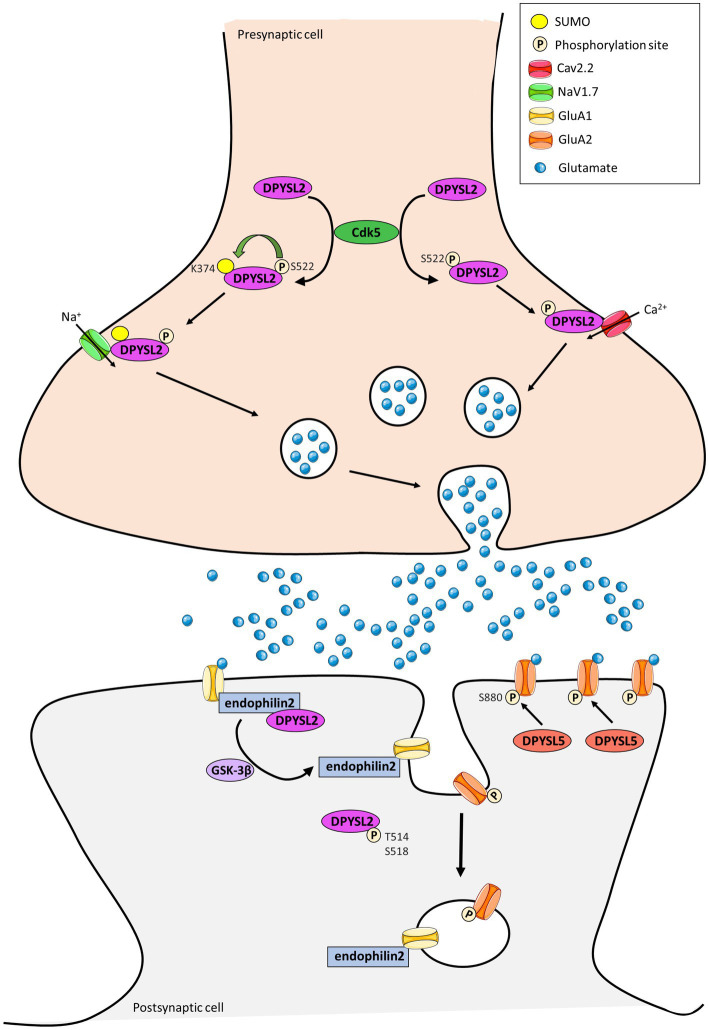
Figure 3.
Representation of the contribution of DPYSL2 and DPYSL5 proteins in the control of synaptic plasticity. DPYSL2 binds and regulates the trafficking of both voltage gated Na2+ (NaV1.7) and Ca2+ (CaV2.2) channels at presynaptic terminal. DPYSL2 phosphorylation at Ser 522 by Cdk5 promotes its binding to Cav2.2. This interaction causes an increased number of CaV2.2 at cell surface leading to an increase in Ca2+ influx and glutamate release. SUMOylation is enhanced by phosphorylation of DPYSL2 through CDK5 action. This SUMOylation induces an increase of NaV1.7 channel at surface and in neuronal excitability. At postsynaptic level, DPYSL2 phosphorylation by GSK3β inhibits interaction with endophilin2 and reduces the number of GluA1 subunits of AMPARs at membrane. Similarly, DPYSL5 via GluA2 S880 phosphorylation can modulate traffic at the surface of the GluA2 subunit of AMPA receptors. (Adapted from Lin et al., 2019; Stratton et al., 2020; Henley et al., 2021).