Severe coronavirus disease 2019 (COVID-19) has been characterized by hyperinflammation, vascular damage, and thrombosis.1,2 Hyperinflammation causes a vasculopathy, particularly in the lungs. Von Willebrand factor (vWF), a marker of the vasculopathy, indeed, is associated with mortality.3,4 Vascular damage triggers activation of the extrinsic pathway of coagulation via exposure of tissue factor (TF). Moreover, activation of the intrinsic pathway of coagulation on the background of neutrophils and neutrophil extracellular trap (NET) formation appears to be key for COVID-19’s hypercoagulability to occur.1,5,6 Yet, the relative contribution of the extrinsic pathway has not been studied in relation to the intrinsic pathway in COVID-19’s hypercoagulability and thus, firm conclusions cannot be drawn. Here, we assessed markers of the extrinsic and intrinsic pathway in a large and well-defined prospective cohort of patients with COVID-19. The dynamics of coagulation were studied during the first wave of the pandemic using a longitudinal design. Also, associations between activation markers of coagulation, vascular damage, and disease severity (i.e., intensive-care unit [ICU] admission, thrombosis, and mortality) were studied.
Consecutive patients with COVID-19 who presented at the Maastricht University Medical Center, Maastricht, the Netherlands, between March 21, 2020, and April 28, 2020, were included. Disease severity was classified as mild (patients not admitted to hospital), moderate (patients admitted to the general ward requiring supplemental oxygen via nasal cannula [≤5 L/min]), and severe (patients requiring supplemental oxygen via a face mask, admitted to the ICU for mechanical ventilation, and/or those who died due to COVID-19). At presentation and at fixed time points during follow-up (i.e., every 5 [±2] days), blood samples were obtained using vacutainer tubes containing 3.2% trisodium citrate and serum tubes; citrated blood was processed immediately and centrifuged at 2,000 g for 10 minutes (min) at room temperature (RT), while serum tubes were allowed to clot for 30 min and centrifuged at 1,885 g for 10 min at RT. Plasma and serum samples were aliquoted and stored at –80°C until testing. Follow-up samples were used when available. Activated coagulation factors in complex with their natural inhibitors (i.e., activated FVII:antithrombin [FVIIa:AT], plasma kallikrein:C1 esterase inhibitor [PKa:C1Inh], FXIa:AT, FXIa:α1-antitrypsin [α1AT], FIXa:AT, and thrombin:antithrombin [T:AT]), desarginated complement 5a (C5a), and vWF:antigen (vWF:Ag), were quantified as described.1,7 Free FVIIa (Staclot VIIarTF; Stago, Asniéres-sur-Seine, France) was quantified according to the manufacturer’s instructions. This study was approved by the appropriate ethics committee (2020-1315), with a waiver of informed consent.
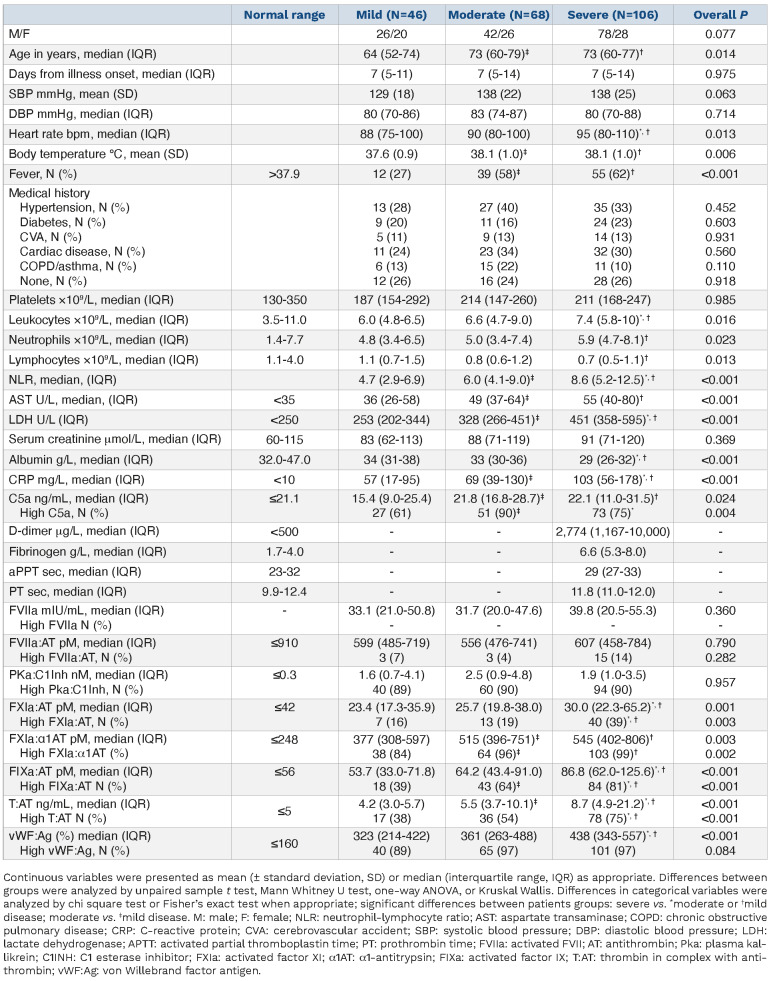
The demographics of the 220 included patients with COVID-19 are depicted in Table 1. Forty-six (21%) patients had mild, 68 (31%) had moderate, and 106 (48%) had severe COVID-19. Thus, 174 patients were admitted, most of whom were treated with antibiotics (n/N=157/174, 90%), chloroquine (n/N=131/174, 75%), and anticoagulation (n/N=154/174, 88%; either prophylactic [n=126] or therapeutic [n=28]) besides oxygen support. At that time, steroids (n/N=6/174, 3%) were not routinely prescribed.
In line with previous studies,8 most patients with severe COVID-19 had elevated levels of D-dimer (n/N=62/64, 97%) and fibrinogen (n/N=55/62, 89%), whereas the activated partial thromboplastin time (n/N=52/71, 73%) and prothrombin time (n/N=56/70, 80%) were often normal (Table 1). Routine coagulation tests were not measured in patients with mild or moderate COVID-19.
We previously demonstrated that T:AT is elevated and linked to disease severity in COVID-19. Neutrophils, NET formation, and activation of the intrinsic pathway drive T:AT levels and COVID-19’s hypercoagulability.1 NET formation, with release of TF, may also activate the extrinsic pathway.9 In order to better understand the interplay and balance between the intrinsic and extrinsic pathways, we assessed markers of the extrinsic pathway. FVIIa:AT, a marker of circulating FVIIa-TF complexes, and free FVIIa did not differ between patients with mild, moderate, or severe COVID-19 and remained stable over time (Table 1; Online Supplementary Table S1). Spearman's ρ indicated a strong positive correlation between T:AT and FXIa:AT (r=0.64; P<0.001) as well as FIXa:AT (r=0.74; P<0.001), but not FVIIa:AT (r=0.14; P=0.097) or free FVIIa (r=0.16; P=0.021; Online Supplementary Figure S1). The role of FVIIa:AT and free FVIIa decreased even more whereas the association of FXI:AT, FIX:AT and T:AT persisted with increasing disease severity, suggesting that FXI activation is mainly caused by FXIIa.10
Activation of the intrinsic pathway may occur on the background of hyperinflammation in COVID-19. FXIa:α1AT (r=0.38; P<0.001), FIX:AT (r=0.41; P<0.001), T:AT (r=0.35, P<0.001), and vWF:Ag (r=0.39; P<0.001) correlated with CRP (Online Supplementary Table S2). We previously showed the intricate link between the intrinsic pathway, neutrophils, and NET formation.1 NET formation, with the release of RNA and DNA, activates the intrinsic pathway.11-13 NET formation, indeed, has been localized to the site of (micro) thrombosis.14 Moreover, NET colocalized with FXII and caused in vitro activation of FXII.5
Table 1.
Baseline characteristics of 220 patients with COVID-19.
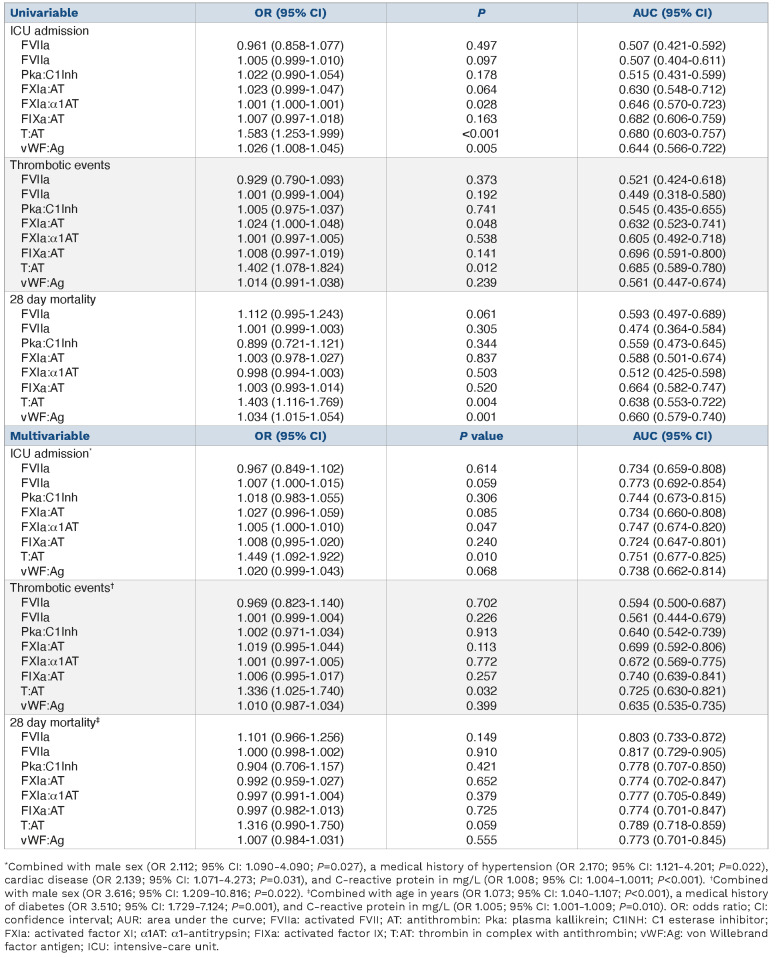
In order to test the prognostic value of activated coagulation factors in complex with their natural inhibitions and vWF:Ag on ICU admission, thrombosis, and mortality, we performed logistic regression analyses (Table 2). Sixty-four (29%) of 220 patients with COVID-19 were admitted to the ICU. FXIa:α1AT, T:AT, and vWF:Ag were associated with an increased risk of ICU admission. This observation remained significant for FXIa:α1AT and T:AT in a multivariable model adjusting for age, CRP, and cardiovascular disease. Henderson et al. corroborated our observations and found that FXIa:AT was associated with progression of lung disease on computed tomography; no data on thrombosis were reported.6 We found thrombosis in 29 (13%; 22 pulmonary embolisms, 3 cerebrovascular accidents, 3 peripheral arterial occlusions, and 1 acute coronary syndrome) of 220 patients with COVID-19, with the highest incidence in patients with severe disease (n=23). Logistic regression linked FXIa:AT and T:AT to thrombosis; T:AT remained significant after adjusting for sex. Fifty-eight (33%) of 174 admitted patients with COVID-19 died in the hospital within 28 days; two patients with moderate disease died because of traumatic brain injury rather than COVID-19 and were excluded from the analysis. Univariable but not multivariable logistic regression linked T:AT and vWF:Ag to in-hospital mortality at 28 days, corroborating previous observations.3,15 Most of these studies, however, were limited because of a cross-sectional design and small sample size. Neither FVIIa:AT nor free FVIIa were associated with clinical outcomes.
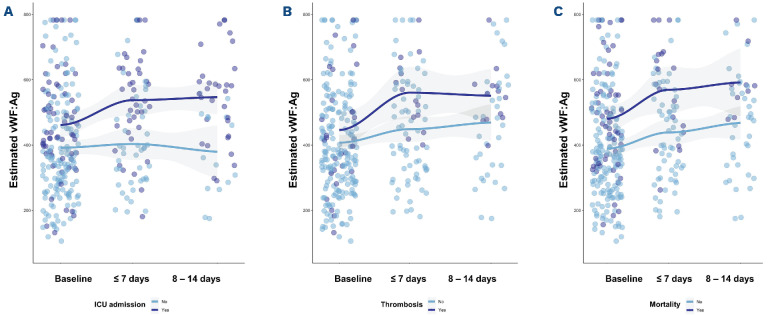
Next, we assessed the prognostic value of activated coagulation factors in complex with their natural inhibitors and vWF:Ag over time in admitted patients using linear mixed models (Figure 1). The dynamics of vWF:Ag were associated with clinical outcomes, whereas none of the activated coagulation markers (i.e., FVIIa:AT, free FVIIa, PKa:C1Inh, FXIa:AT, FXIa:α1AT, FIXa:AT and T:AT) was (data not shown); at presentation, however, FXIa:AT (+85.2; 95% confidence interval [CI]: 13.5-156.8; P=0.020) and T:AT (+9.6 (95% CI: 1.4-18; P=0.023) were higher in patients with thrombotic events. vWF:Ag increased over time, particularly in patients admitted to the ICU and in patients who died. The increase in vWF:Ag was steeper in patients who died as compared to those who survived. Of note, elevated levels of PKa:C1Inh, FXIa:α1AT, FIXa:AT and T:AT remained stable during hospital admission (data not shown). Taken together, ongoing vascular damage, as reflected by vWF:Ag, is associated with poor outcomes, whereas activation of the intrinsic pathway relates to COVID-19’s hypercoagulability and thrombosis. Previous data suggest ongoing activation of the intrinsic pathway, thrombin formation, and vascular damage for up to 3 months after onset of COVID-19.7 Future studies are needed to study the role of coagulation and vascular damage in long COVID-19. Our study has several limitations. First, this cohort was collected at the beginning of the pandemic when thrombotic events were not routinely screened for and thrombosis could have been missed. Second, the limited number of follow-up samples may affect interpretation of data. Our data, however, benefit from the prospective design and inclusion of a large and well-defined cohort of patients with COVID-19. Moreover, our data reflect the natural course of disease because most patients were not treated with glucocorticosteroids and/or immunosuppressive agents.
Figure 1.
Predicted estimates of von Willebrand factor antigen stratified by different clinical outcomes at baseline and over time in patients with COVID-19. Linear mixed-effects models were used to illustrate the effects of von Willebrand factor antigen (vWF:Ag) on intensive-care unit (ICU) admission, thrombosis, and 28 day in-hospital mortality. (A) Estimated vWF:Ag was for ICU admitted patients at baseline +9.0 (95% confidence interval [CI]: -55 to 73; P=0.782) higher than non-ICU admitted patients. Over time, vWF:Ag decreased (-3.0; 95% CI: -27 to 22; P=0.826) overall, but increased significantly in ICU admitted patients (+61; 95% CI: 28-94; P<0.001). (B) Baseline vWF:Ag was comparable between patients with (+7.0; 95% CI: -80 to 93; P=0.879) and without thrombosis. Over time, vWF:Ag increased significantly in both groups (+28; 95% CI: 9.0-48; P=0.005) without a statistical significant difference for patients with thrombosis (+34; 95% CI: -7.0 to 74; P=0.103). (C) vWF:Ag tend to be higher in non-survivors (+45; 95% CI: -28 to 117; P=0.225) and increased over time (+28; 95% CI: 8-47; P=0.006) in both groups with a statistically significant sharper increase in non-survivor (+49; 95% CI: 7-90; P=0.023).
Table 2.
Logistic regression was performed to ascertain the effects of the coagulation factors (per ten units) alone (univariable) and together with other predictors (multivariable) on the likelihood of intensive-care unit admission, thrombosis and 28 day mortality.
In conclusion, we showed that thrombin formation, particularly via the intrinsic pathway, is critical for COVID-19’s hypercoagulability to occur. Thrombin formation and vascular damage are important markers of disease severity, thrombosis, and mortality. The intrinsic pathway may therefore be a potential target for the treatment of this devastating disease. Future studies should address whether our findings can be extrapolated to other (viral) respiratory conditions or not.
Supplementary Material
Funding Statement
Funding: HC and HMHS receive grant support from the Netherlands Heart Foundation (CVON2014-09, Reappraisal of Atrial Fibrillation: Interaction between HyperCoagulability, Electrical Remodeling, and Vascular Destabilization in the Progression of Atrial Fibrillation (RACE V), and from REG-MED XB: Cardiovascular Moonshot. HC was supported by a fellowship of the Gutenberg University Mainz. MV is supported in part by funding from a Health-Holland project/partnership grant (LSHM 9111).
References
- 1.Busch MH, Timmermans S, Nagy M, et al. Neutrophils and contact activation of coagulation as potential drivers of COVID-19. Circulation. 2020;142(18):1787-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinkovits G, Reti M, Muller V, et al. Associations between the von Willebrand Factor-ADAMTS13 axis, complement activation, and COVID-19 severity and mortality. Thromb Haemost. 2022;122(2):240-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vassiliou AG, Keskinidou C, Jahaj E, et al. ICU admission levels of endothelial biomarkers as predictors of mortality in critically Ill COVID-19 patients. Cells. 2021;10(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Englert H, Rangaswamy C, Deppermann C, et al. Defective NET clearance contributes to sustained FXII activation in COVID-19-associated pulmonary thrombo-inflammation. EBioMedicine. 2021;67:103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson MW, Lima F, Moraes CRP, et al. Contact and intrinsic coagulation pathways are activated and associated with adverse clinical outcomes in COVID-19. Blood Adv. 2022;6(11):3367-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willems LH, Nagy M, Ten Cate H, et al. Sustained inflammation, coagulation activation and elevated endothelin-1 levels without macrovascular dysfunction at 3 months after COVID-19. Thromb Res. 2022;209:106-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polimeni A, Leo I, Spaccarotella C, et al. Differences in coagulopathy indices in patients with severe versus non-severe COVID-19: a meta-analysis of 35 studies and 6427 patients. Sci Rep. 2021;11(1):10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skendros P, Mitsios A, Chrysanthopoulou A, et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 2020;130(11):6151-6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naito K, Fujikawa K. Activation of human blood coagulation factor XI independent of factor XII. Factor XI is activated by thrombin and factor XIa in the presence of negatively charged surfaces. J Biol Chem. 1991;266(12):7353-7358. [PubMed] [Google Scholar]
- 11.Kannemeier C, Shibamiya A, Nakazawa F, et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A. 2007;104(15):6388-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noubouossie DF, Whelihan MF, Yu YB, et al. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood. 2017;129(8):1021-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semeraro F, Ammollo CT, Morrissey JH, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118(7):1952-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Middleton EA, He XY, Denorme F, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin X, Duan Y, Bao T, et al. The values of coagulation function in COVID-19 patients. PLoS One. 2020;15(10):e0241329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.