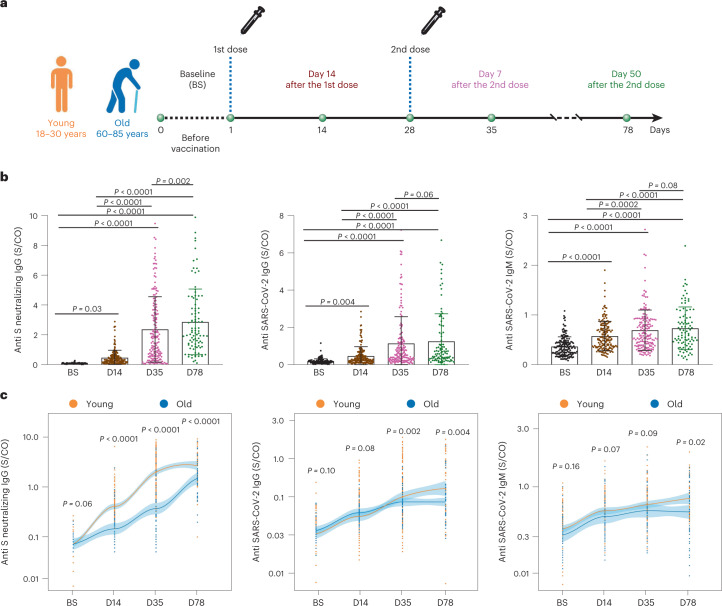
Fig. 1. Study design and statistics of anti-SARS-CoV-2 antibodies in inactivated SARS-CoV-2 vaccine participants.
a, Study design and sample collection timeline. Volunteer participants received two doses of the inactivated SARS-CoV-2 vaccine (CoronaVac or BBIBP-CorV), and blood samples were collected at indicated timepoints. b, SARS-CoV-2 neutralizing IgG, total IgG and IgM quantification by ELISA. The data are presented for all donors. BS, baseline, before vaccination; D14, 14 days after the first dose; D35, 35 days after the first dose, which is 7 days after the second dose; D78, 78 days after the first injection, which is 50 days after the second dose. c, Comparison of SARS-CoV-2 neutralizing antibody titer between young and old donors across the four timepoints. Young: 18–30-year-old healthy donor; Old: 60–85-year-old healthy donor. Significance was assessed by one-way ANOVA corrected for multiple comparisons using the least significant difference (LSD) method. Colored lines were fitted with cross-timepoint averages from each group with shading representing 95% confidence bands. Data are shown as mean ± s.d. n = 169 (121 young and 48 old) for BS, D14 and D35; n = 93 (45 young and 48 old) for D78. For b and c, each dot represents a single individual. P values were determined by one-way ordinary ANOVA.