Abstract
After a skin injury, keratinocytes switch from a state of homeostasis to one of regeneration leading to the reconstruction of the epidermal barrier. The regulatory mechanism of gene expression underpinning this key switch during human skin wound healing is enigmatic. Long noncoding RNAs (lncRNAs) constitute a new horizon in the understanding of the regulatory programs encoded in the mammalian genome. By comparing the transcriptome of an acute human wound and skin from the same donor as well as keratinocytes isolated from these paired tissue samples, we generated a list of lncRNAs showing changed expression in keratinocytes during wound repair. Our study focused on HOXC13-AS, a recently evolved human lncRNA specifically expressed in epidermal keratinocytes, and we found that its expression was temporally downregulated during wound healing. In line with its enrichment in suprabasal keratinocytes, HOXC13-AS was found to be increasingly expressed during keratinocyte differentiation, but its expression was reduced by EGFR signaling. After HOXC13-AS knockdown or overexpression in human primary keratinocytes undergoing differentiation induced by cell suspension or calcium treatment and in organotypic epidermis, we found that HOXC13-AS promoted keratinocyte differentiation. Moreover, RNA pull-down assays followed by mass spectrometry and RNA immunoprecipitation analysis revealed that mechanistically HOXC13-AS sequestered the coat complex subunit alpha (COPA) protein and interfered with Golgi-to-endoplasmic reticulum (ER) molecular transport, resulting in ER stress and enhanced keratinocyte differentiation. In summary, we identified HOXC13-AS as a crucial regulator of human epidermal differentiation.
Subject terms: Epigenetics, Epigenetics, RNA
Introduction
The epidermis is the outermost and stratified epithelium layer, and it protects the human body from external stimuli and prevents dehydration [1]. Keratinocytes constitute ~90% of all epidermal cells and undergo terminal differentiation to form the basal, spinous, granular, and cornified layers of the epidermis. Keratinocyte differentiation has been characterized by a dynamically changed gene expression program, e.g., early differentiated keratinocytes express KRT1, KRT10, and IVL, and then, LOR and FLG levels increase at later differentiation stages [2, 3]. Well-balanced keratinocyte proliferation and differentiation are essential for maintaining epidermal homeostasis, which is disrupted by skin injury, and wound-edge keratinocytes swiftly switch their status to engage in regeneration [4]. The dynamic gene expression and related regulatory mechanisms underpinning the switch between keratinocytes in the epidermal homeostasis state and the regeneration state are not fully understood; the mechanism is even more elusive in the human tissue environment during wound healing. Addressing fundamental questions about homeostasis-to-regeneration phenotype switching is required to understand the pathological mechanism underlying failed re-epithelization in chronic nonhealing wounds, which have led to major and increasing health and financial burdens worldwide [5, 6].
In addition to protein-coding genes, most of the human genome comprises a vast landscape of regulatory elements, including tens of thousands of long noncoding RNAs (lncRNAs). LncRNAs are transcripts longer than 200 nucleotides with no or limited sequences that can be translated [7, 8]. Increasing numbers of lncRNAs have been shown to regulate vital cellular processes via a large variety of molecular mechanisms and to play critical roles in health and disease [9]. Importantly, both the expression pattern of lncRNAs and their functions are more cell-type- and cell-state-specific than protein-coding genes, which endows lncRNAs with promising therapeutic and diagnostic potential [10–12]. In the skin, a few lncRNAs, including ANCR, TINCR, LINC00941, uc.291, and PRANCR, have been shown to regulate keratinocyte differentiation [13–17]. Moreover, three lncRNAs have been reported to function in keratinocytes during skin wound healing, i.e., WAKMAR2 suppresses the inflammatory response, while WAKMAR1, WAKMAR2, and TETILA change cell mobility [18–20]. Although in its infancy, this field has produced evidence suggesting that lncRNAs are important regulators in epidermal homeostasis and regeneration, and further efforts to gain a more holistic and deeper understanding of these RNAs are warranted.
In this study, by tracing the in vivo transcriptomic changes in keratinocytes during human skin wound healing, we generated a list of lncRNAs that changed during the switch between the epidermal homeostasis state and the regeneration state. We focused on a human skin- and keratinocyte-specific lncRNA, HOXC13-AS, and revealed the crucial role it plays in regulating keratinocyte differentiation by interfering with retrograde protein transport from the Golgi to the endoplasmic reticulum (ER). The temporal downregulation of HOXC13-AS in wound-edge keratinocytes, likely due to high EGFR signaling during wound repair, and its restored expression during re-epithelialization reflect its physiological importance in the maintenance and reconstruction of the epidermal barrier.
Results
Downregulation of HOXC13-AS expression in human wound-edge keratinocytes
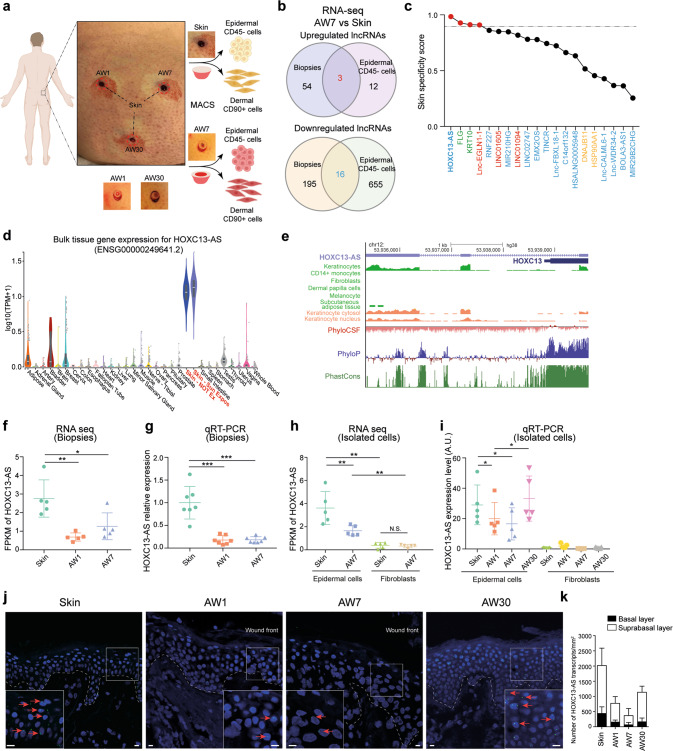
To characterize the role played by lncRNAs in human skin wound healing, we created wounds on the skin of healthy donors and collected the wound-edge tissues on day 1 (acute wound day 1, AW1), seven (AW7), and 30 (AW30) until the wounds closed (Fig. 1a). With ribosomal RNA-depleted long RNA-sequencing (RNA-seq) of these full-thickness tissue biopsy samples, we identified 57 upregulated and 211 downregulated lncRNAs on AW7 compared to the expression of these lncRNAs in matched skin from the five donors [|fold change| ≥ 2, False discovery rate (FDR) < 0.05, Fig. 1b and Supplementary Data 1]. To detect keratinocyte-related lncRNA expression changes, we conducted RNA-seq with epidermal CD45- cells and identified 15 upregulated and 671 downregulated lncRNAs in keratinocytes isolated from the AW7 wound edges compared to expression in the matched skin samples (|fold change| ≥ 2, FDR < 0.05, Fig. 1b and Supplementary Data 2). Interestingly, 3 upregulated and 16 downregulated lncRNAs were identified in both the tissue and epidermal cell RNA-seq analyses (Fig. 1b). We ranked these 19 lncRNAs on the basis of their skin specificity scores, which were calculated by the SPECS method [21] with the RNA-seq data obtained from 31 human normal tissues in the GTEx database [22]. HOXC13 antisense RNA (HOXC13-AS) was identified as a skin-specific lncRNA, with a score even higher than some known skin-specific genes, e.g., FLG and KRT10 (Fig. 1c, d). The highly specific expression pattern of HOXC13-AS suggests its potentially unique function in the skin, which prompted us to take a closer look at this lncRNA.
Fig. 1. Downregulation of HOXC13-AS expression in human wound-edge keratinocytes.
a Human in vivo wound model: full-thickness excisional wounds were created on the skin of healthy volunteers and wound-edge tissues were collected 1 (AW1), 7 (AW7), and 30 days later (AW30) from the same donor. Epidermal CD45− cells (enriched with keratinocytes) and dermal CD90+ cells (fibroblasts) were isolated from the skin and AW7 biopsies by magnetic-activated cell sorting (MACS). RNA-sequencing was performed in both the tissues and the cells. b Venn diagram showing the differentially expressed lncRNAs in the CD45− epidermal cells and the tissue biopsies of the AW7 compared to the skin (|Fold change| ≥ 2, p < 0.05). c Skin specificity scores of the differentially expressed lncRNAs surfaced in both the tissue and the epidermal cell RNA-seq analysis (upregulated in red, downregulated in blue), known skin-specific genes KRT10 and FLG (green), and broadly expressed genes DNAJB11 and HSP90AA1 (yellow). The dashed line indicates the skin specificity score of 0.9. d HOXC13-AS expression data across 31 normal human tissues retrieved from the GTEx database. e Genomic snapshot of HOXC13-AS generated in GENECODE V38. Data were retrieved from Encyclopedia of DNA Elements data hub, phylogenetic information-based codon substitution frequency (PhyloCSF), and conservation tracks (PhyloP and PhastCons). RNA-seq of HOXC13-AS in tissue biopsies (n = 5 donors) (f) and isolated cells (n = 5 donors) (h). Data are normalized as Fragments per kilobase of a transcript, per million mapped reads (FPKM). QRT-PCR analysis of HOXC13-AS in tissue biopsies (n = 7 donors) (g) and isolated cells (n = 5 donors) (i). Representative photographs (j) and quantification (k) of HOXC13-AS fluorescence in situ hybridization (FISH) in human skin and wounds (n = 2 donors). Cell nuclei were co-stained with DAPI (scale bar = 10 μm). *p < 0.05; **p < 0.01; ***p < 0.001 by Mann–Whitney test (f–h) and paired two-tailed Student’s t test (i). Data are presented as mean ± SD.
HOXC13-AS is a divergent lncRNA transcribed from the opposite strand of the protein-coding gene HOXC13 on human chromosome 12 [GRCh38/hg38, chr12:53,935,328–53,939,643] (Fig. 1e and Supplementary Fig. 1a). It is a recently evolved human lncRNA, as indicated by the low PhyloP and PhastCons scores, and no homolog in rodents has been identified. Moreover, a phylogenetic information-based codon substitution frequency analysis (PhyloCSF) suggested that HOXC13-AS lacks protein-coding potential, which was in agreement with a coding potential calculator CPC2 analysis [23] showing that the coding potential of HOXC13-AS was even lower than that of HOTAIR [24], a well-known lncRNA (Supplementary Fig. 1b).
Our RNA-seq analysis of human wounds revealed that HOXC13-AS expression was downregulated by AW1 and remained low through AW7 compared to that in the matching skin, and these findings were confirmed by real-time RT–PCR (qRT–PCR) analysis of tissue biopsy samples from seven other donors (Fig. 1f, g). Moreover, we performed RNA-seq and qRT–PCR with paired epidermal keratinocytes and dermal fibroblasts isolated from skin and wound samples on AW7 from ten healthy donors. We found that in human skin, HOXC13-AS was mainly expressed in keratinocytes but not in fibroblasts (Fig. 1h, i), which was in line with the public ENCODE data regarding HOXC13-AS expression in different cell types (Fig. 1e). Keratinocyte HOXC13-AS expression was transiently downregulated on AW1 and AW7 and then recovered to the level in paired skin on AW30, at which point the wounds had re-epithelized and showed epidermal stratification (Fig. 1i). Performing fluorescence in situ hybridization (FISH), we not only confirmed these findings but also localized HOXC13-AS mainly in the suprabasal layers of the epidermis (Fig. 1j, k and Supplementary Fig. 1e). Additionally, in published RNA-seq datasets of human wounds, we found reduced HOXC13-AS expression in diabetic foot ulcers compared to diabetic foot skin [25] and on day 2 and 5 in acute wounds compared to the expression in the skin [26] (Supplementary Fig. 1c, d). Consistent with its skin-specific expression (Fig. 1c, d), HOXC13-AS was not detected in human oral mucosal wounds [26] (Supplementary Fig. 1d).
In summary, we identified a human skin keratinocyte-specific lncRNA: HOXC13-AS. The expression of this lncRNA was reduced upon skin injury but was restored during re-epithelization, suggesting that HOXC13-AS may be involved in the maintenance and reconstruction of the epidermal barrier.
Single-cell transcriptomic analysis of human skin revealed increased HOXC13-AS expression in granular keratinocytes
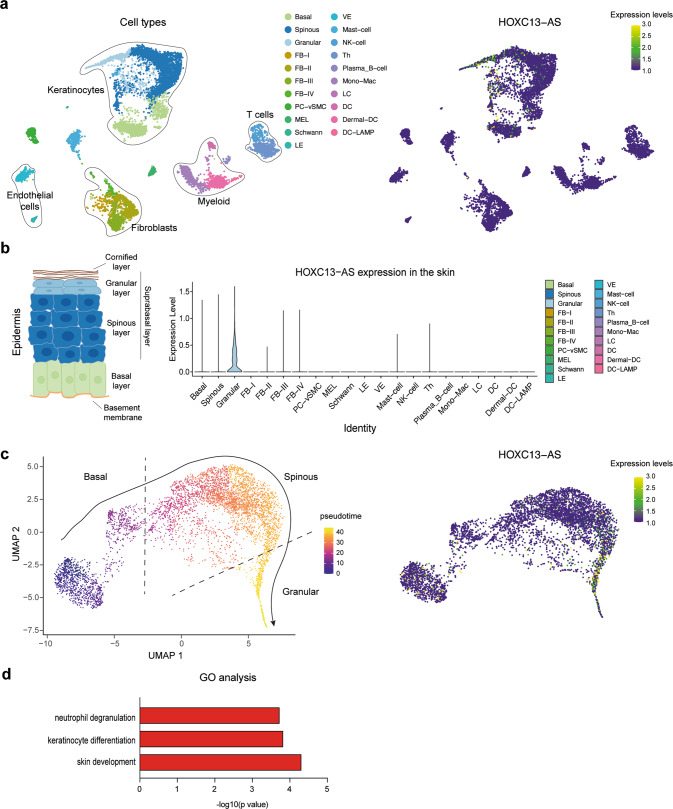
We performed a single-cell RNA-sequencing (scRNA-seq) analysis of human skin (n = 3) using 10X Chromium technology. Unsupervised clustering of 11800 cells with the Seurat package revealed 21 cell clusters, which included keratinocytes (basal, spinous, and granular keratinocytes), fibroblasts (FB-I–IV), melanocytes (MELs), Schwann cells, pericytes-vascular smooth muscle cells (PC-vSMCs), lymphatic and vascular endothelial cells (LE and VE), and immune cells [mast cells, NK cells, B cells, monocytes-macrophages (Monos-Macs), Langerhans cells (LC), and dendritic cells (DC)] (Fig. 2a). In line with the bulk RNA-seq data obtained with individual cell types (Fig. 1e, h), scRNA-seq demonstrated keratinocyte-specific HOXC13-AS expression in human skin (Fig. 2a). Interestingly, we found that HOXC13-AS was predominantly expressed in granular keratinocytes (Fig. 2b), which agreed with our FISH results showing higher HOXC13-AS expression in the suprabasal layers of the epidermis (Fig. 1j, k and Supplementary Fig. 1e). Additionally, a pseudotime analysis showed that all skin keratinocytes were ordered along a differentiation trajectory, which revealed that HOXC13-AS expression increased with keratinocyte differentiation (Fig. 2c). Notably, epidermal cell differentiation has been shown to be reduced during wound repair [27], which was confirmed here by analyzing late differentiation marker FLG expression in human acute wounds with immunofluorescence (IF) staining, as well as single-cell and spatial transcriptomic analysis, showing decreased FLG expression in wound-edge granular keratinocytes (Supplementary Fig. 1f–h). Therefore, HOXC13-AS expression changes with keratinocyte differentiation also during wound repair. Furthermore, to envisage the potential functional role of HOXC13-AS, we performed an expression correlation analysis between HOXC13-AS and all other genes expressed in the skin granular keratinocytes examined by the scRNA-seq, and thus, we identified 1935 positively correlated genes (R > 0, p < 0.05). Gene Ontology analysis (GO) unraveled that the 50 genes most correlated with HOXC13-AS were mainly involved in keratinocyte differentiation and immune response, suggesting that this lncRNA may play a role in these biological processes (Fig. 2d and Supplementary Data 3).
Fig. 2. Granular keratinocytes are the major cells expressing HOXC13-AS in the skin.
a UMAP representation of all cell types (left) and HOXC13-AS expressing cells (right) identified in human skin (n = 3 donors) by single-cell RNA-sequencing (scRNA-seq). b Violin plots of HOXC13-AS expression in different cell types in human skin. c Pseudotime trajectory of all the keratinocytes (i.e., basal, spinous, and granular keratinocytes) colored by pseudotime (left) and HOXC13-AS expression (right). d Gene Ontology (GO) analysis of the top 50 genes with expression positively correlated with HOXC13-AS in granular keratinocytes analyzed by scRNA-seq.
HOXC13-AS expression is regulated in the opposite direction by keratinocyte growth and differentiation signaling
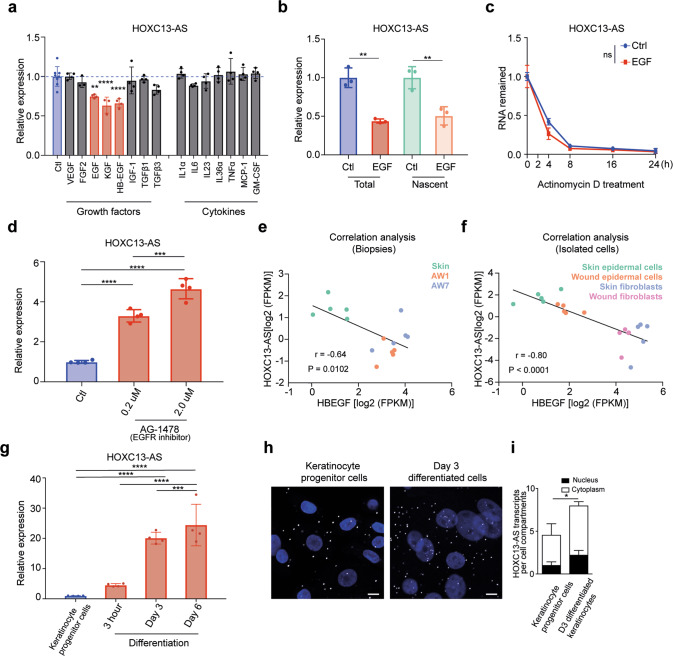
We next explored the mechanism that modulates HOXC13-AS expression in epidermal homeostasis and regeneration. We treated human keratinocytes with a panel of growth factors (VEGF-A, FGF2, EGF, KGF, HB-EGF, IGF-1, TGF-β1, and TGF-β3) and cytokines (IL-1α, IL-6, IL-23, IL-36α, TNFα, MCP-1, and GM-CSF) known to be important to wound repair [28]. A qRT-PCR analysis revealed that HOXC13-AS expression was significantly downregulated by two members of the EGF family (HB-EGF and EGF) [28] and KGF (Fig. 3a and Supplementary Fig. 2a). Additionally, reduced HOXC13-AS expression was found in a public dataset (GSE156089) generated with an RNA-seq analysis of epidermal stem cells treated with EGF (Supplementary Fig. 2b). By labeling and purifying nascent RNA and then qRT-PCR detection of newly transcribed HOXC13-AS, we showed that EGF treatment reduced HOXC13-AS transcription in keratinocytes (Fig. 3b). Moreover, after blocking transcription with Actinomycin D, we found that EGF did not change HOXC13-AS stability (Fig. 3c). These data suggest that EGF may regulate HOXC13-AS expression at the transcriptional level. Furthermore, we showed that blocking EGF receptor (EGFR) signaling with the chemical inhibitor AG1478 [29] prominently enhanced HOXC13-AS expression in keratinocytes in a dose-dependent manner, which confirmed the inhibitory effect of growth factor signaling on HOXC13-AS expression in keratinocytes (Fig. 3d). Importantly, we found that the expression of HB-EGF and KGF was significantly upregulated during human skin wound repair and a significantly negative correlation between HOXC13-AS and HBEGF expression, as shown by the RNA-seq analysis of human wound tissues and isolated cells, suggesting that enhanced growth factor signaling may contribute to the downregulation of HOXC13-AS expression in human wounds in vivo (Fig. 3e, f and Supplementary Fig. 2c, d).
Fig. 3. HOXC13-AS expression is regulated in the opposite direction by keratinocyte growth and differentiation signaling.
a QRT-PCR analysis of HOXC13-AS expression in keratinocytes treated with wound-related cytokines and growth factors for 24 h (n = 4). b QRT-PCR analysis of total and nascent HOXC13-AS in keratinocytes treated with EGF for 8 h (n = 3). c QRT-PCR analysis of HOXC13-AS in keratinocytes treated with EGF and then actinomycin D for 0–24 h (n = 4). d QRT-PCR analysis of HOXC13-AS in keratinocytes treated with AG-1478 for 24 h (n = 4). Expression correlation of HOXC13-AS with HBEGF in the skin and wound tissues (e) and the isolated cells (f) analyzed by RNA-seq. g QRT-PCR analysis of HOXC13-AS in keratinocyte progenitor cells and calcium-induced differentiated keratinocytes (n = 4). Representative photographs (h) and quantification (i) of HOXC13-AS FISH in keratinocyte progenitor cells and differentiated keratinocytes treated with 1.5 mM calcium for 3 days (n = 3). Cell nuclei were co-stained with DAPI. Scale bar = 20 μm. ns not significant, *p < 0.05; **p < 0.01, ***p < 0.001 and ****p < 0.0001 by unpaired two-tailed Student’s t test (a, b, d, g, i), two-way ANOVA (c), or Pearson’s correlation test (e and f). Data are presented as mean ± SD.
As the FISH and scRNA-seq analyses of human skin showed higher HOXC13-AS expression in more-differentiated keratinocytes (Figs. 1j, k and 2c) and because EGFR signaling inhibition has been reported to induce keratinocyte differentiation [30], we next examined whether differentiation enhanced HOXC13-AS expression in keratinocytes. Studying a calcium-induced keratinocyte differentiation model [31, 32], we found that HOXC13-AS expression gradually increased as cell differentiation progressed, as shown by both HOXC13-AS qRT–PCR and FISH analysis results (Fig. 3g–i and Supplementary Fig. 2e, f), which was also confirmed in a public transcriptomic analysis of differentiated keratinocytes at multiple time points (GSE59827) [33] (Supplementary Fig. 2g). Notably, differentiation also slightly reduced EGFR expression in keratinocytes, which may contribute to the differentiation-induced HOXC13-AS expression (Supplementary Fig. 2g, h).
Considering these data, we concluded that HOXC13-AS expression in keratinocytes was induced by cell differentiation but suppressed by growth factor signaling, which explains its increased expression in the suprabasal layers of the epidermis that contain more differentiated keratinocytes and its decreased expression during wound repair likely due to high growth signaling and decreased differentiation.
HOXC13-AS promotes keratinocyte differentiation
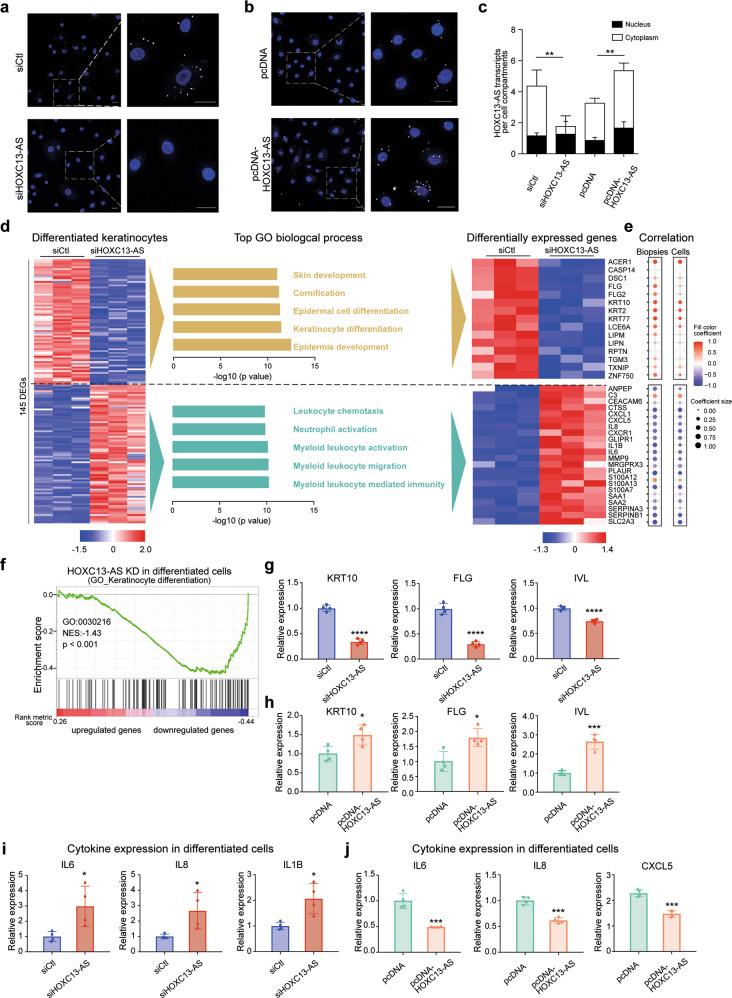
To characterize the potential functional role played by HOXC13-AS, we knocked down (KD) HOXC13-AS expression by transfecting human keratinocytes with HOXC13-AS-specific short interfering RNAs (siRNAs), and the loss of HOXC13-AS was confirmed by FISH (Fig. 4a, c) and qRT–PCR analyses (Supplementary Fig. 3a). In addition, we overexpressed (OE) HOXC13-AS in keratinocytes using a HOXC13-AS expression vector (pcDNA-HOXC13-AS), which significantly increased HOXC13-AS levels in the keratinocytes (Fig. 4b, c and Supplementary Fig. 3b). We showed that neither HOXC13-AS KD nor OE affected HOXC13 expression in progenitor or differentiated keratinocytes (Supplementary Fig. 3c–e).
Fig. 4. HOXC13-AS regulates keratinocyte differentiation and inflammatory response.
Representative photographs (a, b) and quantification (c) of HOXC13-AS FISH in keratinocytes transfected with HOXC13-AS siRNA pool or nontargeting control (siCtl) (a), pcDNA-HOXC13-AS or empty vector (b) (n = 3). Cell nuclei were co-stained with DAPI. Scale bar = 20 μm. d Microarray analysis of differentiated keratinocytes transfected with HOXC13-AS siRNA pool or siCtl. Heatmap (left panel) illustrates the differentially expressed genes (DEGs, |Fold change| ≥ 2, FDR < 0.05). GO analysis of the DEGs is shown in the middle panel. The DEGs associated with the GO terms are shown in the heatmap (right panel). e Their expression correlation with HOXC13-AS in the skin and wound biopsies and isolated epidermal cells analyzed by RNA-seq. f GSEA evaluated enrichment for the keratinocyte differentiation-related genes (GO:0030216) in the microarray data. NES normalized enrichment score. QRT-PCR analysis of KRT10, FLG and IVL expression in differentiated keratinocytes transfected with HOXC13-AS siRNA pool (g) or pcDNA-HOXC13-AS (h) compared to respective controls (n = 4). QRT-PCR analysis of cytokine expression in differentiated keratinocytes transfected with HOXC13-AS siRNA pool (i) or pcDNA-HOXC13-AS (j) compared to respective controls (n = 4). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 by unpaired two-tailed Student’s t test (c, g–j), or Pearson’s correlation test (e). Data are presented as mean ± SD.
Next, we performed a microarray analysis with calcium-induced differentiated keratinocytes in which HOXC13-AS expression was knocked down, which led to the identification of 76 upregulated and 69 downregulated genes (|fold change| ≥ 2, p value < 0.05) (Fig. 4d). A GO analysis revealed that the expression of genes involved in keratinocyte differentiation (e.g., KRT10, FLG, DSC1 [34], and CASP14 [35]) was decreased, whereas the expression of immune response-related genes (e.g., IL6, IL8, and IL1B) was increased after HOXC13-AS was knocked down (Fig. 4d). Furthermore, we evaluated the expression of these HOXC13-AS-regulated genes in our RNA-seq datasets of human wound tissues and isolated epidermal cells (Fig. 1a). We found that differentiation- and inflammation-related genes were positively and negatively correlated with HOXC13-AS levels, respectively, supporting the in vivo relevance of our findings to HOXC13-AS-mediated gene regulation (Fig. 4e and Supplementary Fig. 3f–i). Additionally, a gene set enrichment analysis (GSEA) [36] confirmed that among the downregulated genes after HOXC13-AS was knocked down, keratinocyte differentiation-related genes (GO: 0030216) were significantly (p < 0.001) enriched (Fig. 4f). Furthermore, we confirmed the microarray findings by performing a qRT–PCR analysis of the keratinocyte differentiation markers (KRT10, FLG, and IVL) and the inflammatory genes (IL6, IL8, IL1B, CXCL5, CXCL1, and MMP9) in a calcium-induced keratinocyte differentiation model with HOXC13-AS OE or KD (Fig. 4g–j and Supplementary Fig. 3j). Both gain- and loss-of-function studies suggested that HOXC13-AS promoted keratinocyte differentiation while suppressing the cellular inflammatory response. Additionally, we showed that neither knocking down nor overexpressing HOXC13-AS affected human progenitor keratinocyte proliferation or migration (Supplementary Fig. 4a–c).
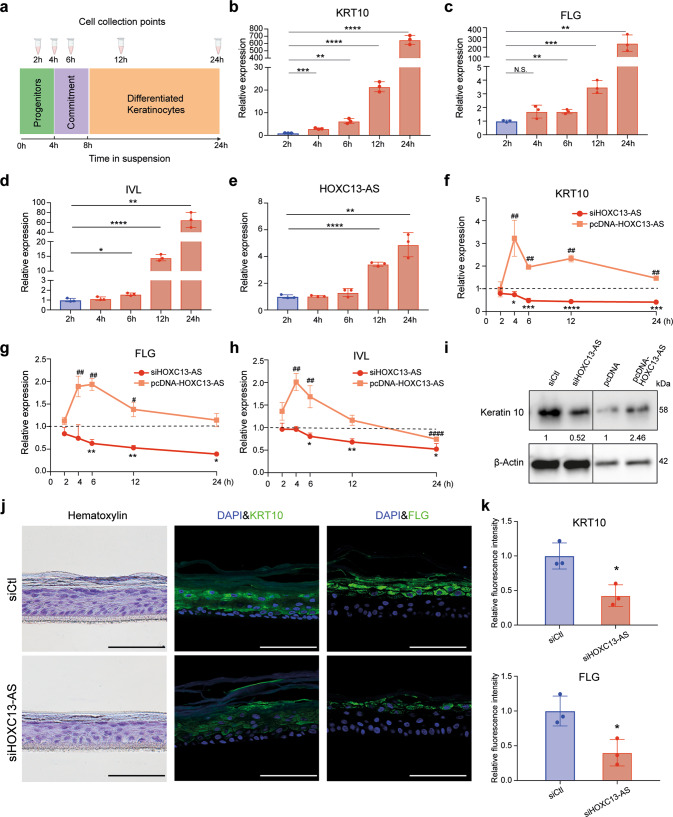
Moreover, we utilized a suspension-induced keratinocyte differentiation model (Fig. 5a) [37, 38]. Human progenitor keratinocytes were cultured in a single-cell suspension from 2 to 24 h, which induced cell differentiation, as shown by the gradually increased expression of the differentiation markers KRT10, FLG, and IVL (Fig. 5b–d). Additionally, HOXC13-AS expression was enhanced through cell differentiation (Fig. 5e). After confirming the high efficiency of HOXC13-AS KD and OE in this model (Supplementary Fig. 4d), we showed that HOXC13-AS OE led to enhanced keratinocyte differentiation, whereas HOXC13-AS KD profoundly reduced differentiation, as evidenced by the changes in KRT10, FLG, and IVL at the mRNA (Fig. 5f–h) and protein levels (Fig. 5i).
Fig. 5. HOXC13-AS regulates keratinocyte differentiation in a suspension-induced differentiation model and organotypic human epidermal tissues.
a Schematic representation of the experimental flow (left panel) and b–e qRT-PCR analysis of KRT10, FLG, IVL, and HOXC13-AS expression in the suspension-induced keratinocyte differentiation model (n = 3). QRT-PCR analysis of KRT10 (f), FLG (g), and IVL (h) in keratinocytes transfected with HOXC13-AS siRNA pool, pcDNA-HOXC13-AS followed by suspension-induced differentiation. The results were normalized with the respective controls (n = 3). i Western blot of Keratin 10 in keratinocytes transfected with HOXC13-AS siRNA pool, pcDNA-HOXC13-AS, or respective controls after suspension induction for 24 h. j Representative photograph of hematoxylin and immunofluorescence staining of the organotypic epidermis with HOXC13-AS knockdown. Cell nuclei were co-stained with DAPI. Scale bar = 100 μm. k Quantification of fluorescence intensities of KRT10 and FLG in organotypic epidermis with HOXC13-AS knockdown (n = 3). * or #p < 0.05; ** or ##p < 0.01, *** or ###p < 0.001 and **** or ####p < 0.0001 by unpaired two-tailed Student’s t test (b–h, k). Data are presented as mean ± SD.
To functionally characterize HOXC13-AS in a tissue environment, we generated organotypic human epidermal tissues [16] using progenitor keratinocytes with HOXC13-AS KD or OE. By performing IF staining, we showed that the abundance of the differentiation markers KRT10 and FLG, but not IVL, in suprabasal layer keratinocytes was significantly reduced after HOXC13-AS KD, whereas KRT10 and FLG expression were enhanced by HOXC13-AS OE, suggesting that modulation of HOXC13-AS changed keratinocyte differentiation in the organotypic epidermis (Fig. 5j, k and Supplementary Fig. 5a, b). Notably, in these organotypic tissues, we did not detect apoptotic cells through IF of caspase 3 (Supplementary Fig. 5c). The number of proliferating keratinocytes, which were localized to the basal layer of the epidermis, as indicated by Ki67 IF staining, was not changed by HOXC13-AS KD (Supplementary Fig. 5d).
Collectively, these data obtained with multiple physiologically relevant keratinocyte differentiation models demonstrated a prominent role for HOXC13-AS in promoting keratinocyte differentiation and showed that HOXC13-AS upregulation is required for maintaining the epidermal barrier.
HOXC13-AS interacts with COPA protein
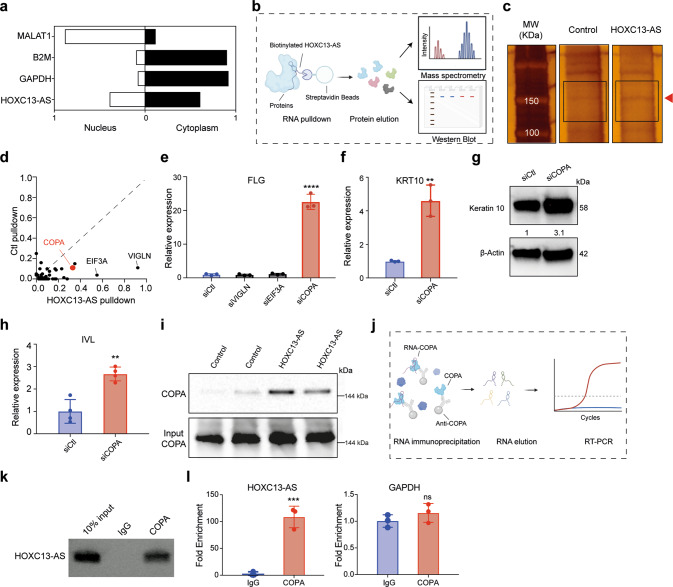
We next investigated the molecular mechanisms mediating the pro-differentiation function of HOXC13-AS in keratinocytes. In this analysis, subcellular localization is usually an indicator of the modes of action of lncRNAs. By performing cell fractionation assays, we showed a greater abundance of HOXC13-AS in the cytoplasm than in the nucleus of keratinocytes (Fig. 6a), which was consistent with our FISH analysis of human skin, wound tissues, and keratinocytes (Figs. 1j, 3h, i and 4a, b and Supplementary Fig. 1e), as well as the public ENCODE data (Fig. 1e). The cytoplasmic localization of HOXC13-AS indicated that it might act via an in trans mode [9, 39].
Fig. 6. HOXC13-AS interacts with COPA protein to regulate keratinocyte differentiation.
a QRT-PCR analysis of HOXC13-AS, GAPDH, B2M, and MALAT1 in the nuclear or cytoplasmic fractions of keratinocytes. b Schematic representation of the RNA pulldown experiment. c Silver staining of proteins bound to HOXC13-AS or the control Poly(A)25 RNA. The red arrow indicates the differential band, and the rectangles specify the gel regions sent for mass spectrometry analysis (MS). d HOXC13-AS bound proteins identified by MS. e QRT-PCR analysis of FLG expression in differentiated keratinocytes with HDLBP, EIF3A, or COPA expression silencing (n = 3). QRT-PCR (f) and western blot (g) of Keratin 10 expression in differentiated keratinocytes with COPA knockdown (n = 3). h QRT-PCR of IVL expression in differentiated keratinocytes with COPA knockdown (n = 3). i Western blot of COPA bound to HOXC13-AS or control RNA. Same amounts of protein lysates were used as the input. j Schematic overview of the RNA immunoprecipitation assays (RIP). k Agarose gel electrophoresis of HOXC13-AS RT–PCR products with the RNA retrieved from RIP. l QRT-PCR analysis of HOXC13-AS and GAPDH retrieved from RIP using COPA antibody or IgG (n = 3). ns not significant, **p < 0.01, ***p < 0.001, and ****p < 0.0001 by unpaired two-tailed Student’s t test (e, f, h, and l). Data are presented as mean ± SD.
We surveyed the protein interactome of HOXC13-AS by performing an RNA pull-down experiment. After incubating keratinocyte protein lysates, biotinylated HOXC13-AS together with its binding proteins were purified in a process based on streptavidin beads [40] (Fig. 6b). Running the purified proteins in a gel followed by silver staining, we observed that a band representing ~150 kDa was more intense in the HOXC13-AS pull-down fraction than in the control poly(A)25 RNA pull-down fraction (Fig. 6c). We excised the regions of this differential band and analyzed the extracted protein by mass spectrometry (MS). High-density lipoprotein binding protein (VIGLN), eukaryotic translation initiation factor 3 subunit A (EIF3A), and coatomer subunit alpha (COPA) were identified as the proteins most enriched in the HOXC13-AS pull-down product fraction (Fig. 6d and Supplementary Data 4). As HOXC13-AS promotes keratinocyte differentiation, we next examined whether any of the identified proteins were involved in the same biological processes. By silencing their expression in keratinocytes with gene-specific siRNAs, we found that only knocking down COPA expression significantly changed FLG expression in differentiated keratinocytes (Fig. 6e). Furthermore, our qRT–PCR and western blotting analyses revealed that COPA silencing induced KRT10 and IVL expression (Fig. 6f–h). Besides the calcium-induced differentiation model, we also confirmed that COPA silencing increased KRT10, FLG, and IVL expression in the suspension-induced keratinocyte differentiation model (Supplementary Fig. 6a). Therefore, our study focused on the COPA protein. We first confirmed the specific pull-down of the COPA protein by HOXC13-AS by western blotting with a COPA-specific antibody (Fig. 6i). Next, we performed RNA immunoprecipitation (RIP) (Fig. 6j), which showed that HOXC13-AS, but not GAPDH, was pulled down by an anti-COPA antibody but not by IgG (Fig. 6k, l and Supplementary Fig. 6b). These two-way ribonucleoprotein complex pull-down experiments provided strong evidence of the binding between HOX1C3-AS and COPA. Notably, silencing HOXC13-AS did not change COPA protein or mRNA levels in keratinocytes (Supplementary Fig. 6c, d), which was in line with our observation that COPA was evenly expressed across different epidermal layers in human skin (Supplementary Fig. 6e, f).
In summary, our data suggested that COPA negatively regulates keratinocyte differentiation and its binding with HOXC13-AS may serve as a key link in the mechanisms controlling epidermal differentiation.
HOXC13-AS interferes with Golgi-ER retrograde transport causing ER stress
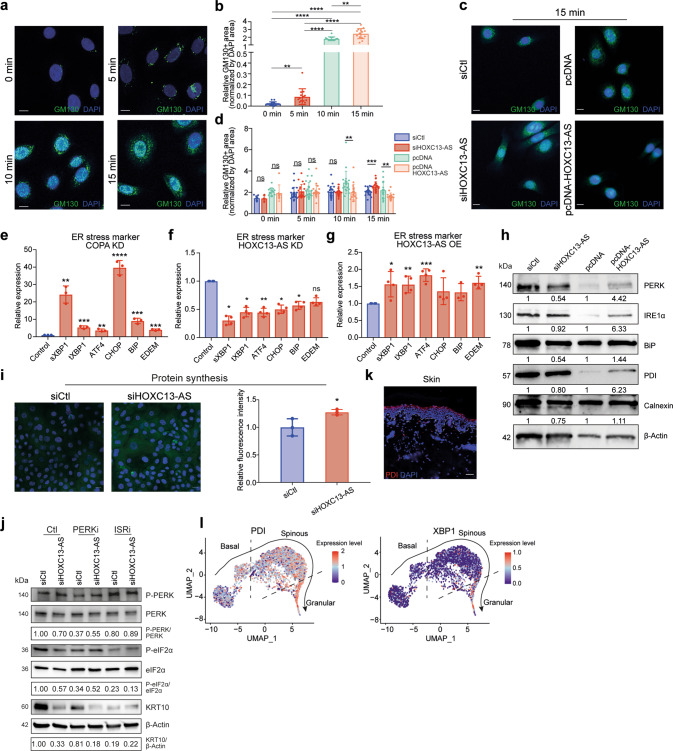
The COPA protein makes up a part of coatomer protein complex I (COPI), which is required for the retrograde transport of cargo proteins from the Golgi to the endoplasmic reticulum (ER) and the movement of vesicles within the Golgi [41]. As HOXC13-AS binds to COPA protein, we next evaluated whether HOXC13-AS affected the COPI-mediated retrograde transport in keratinocytes. To this end, we utilized brefeldin A (BFA), a fungal metabolite that blocks ER-to-Golgi transport [42], and assessed the extent of the redistribution of the Golgi marker GM130 from the Golgi to the ER [43]. Consistent with previous studies [44], GM130 IF showed that BFA treatment quickly induced Golgi-ER retrograde transport in keratinocytes (Fig. 7a, b). Interestingly, we found that HOXC13-AS KD enhanced and HOXC13-AS OE reduced GM130 retrograde transport (Fig. 7c, d and Supplementary Fig. 7a–c).
Fig. 7. HOXC13-AS interferes with Golgi-ER retrograde transport causing ER stress.
Representative photograph (a) and quantification (b) of immunofluorescence staining of GM130 in keratinocytes treated with Brefeldin A for 0–15 min. Cell nuclei were co-stained with DAPI. (Scale bar = 10 μm, n = 14–15 cells). Representative photograph (c) and quantification (d) of immunofluorescence staining of GM130 in keratinocytes transfected with HOXC13-AS siRNA pool, pcDNA-HOXC13-AS or respective controls, and treated with Brefeldin A. Cell nuclei were co-stained with DAPI (scale bar = 10 μm, n = 8–13 cells at 0 min and n = 18–22 cells at 5, 10, and 15 min). QRT-PCR analysis of ER stress markers in differentiated keratinocytes with COPA knockdown (KD, n = 3) (e), HOXC13-AS KD (n = 4) (f), or HOXC13-AS overexpression (OE, n = 4) (g). h Western blot of ER stress markers in keratinocytes transfected with HOXC13-AS siRNA pool, pcDNA-HOXC13-AS or respective controls after suspension induction for 24 h. i Protein synthesis assay in differentiated keratinocytes with HOXC13-AS KD (n = 3). j Western blot in differentiated keratinocytes with HOXC13-AS KD and treated with PERK inhibitors (PERKi) or inhibitors of the integrated stress response (ISRi). k Immunofluorescence staining of PDI in human skin. Cell nuclei were co-stained with DAPI. Scale bar = 50 μm. l PDI and XBP1 expression in pseudotime trajectory of keratinocytes in human skin analyzed by single-cell RNA-seq. ns not significant, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 by unpaired two-tailed Student’s t test (b, d–g, and i). Data are presented as mean ± SD.
COPA mutations have been shown to cause ER stress that triggers the unfolded protein response (UPR) [45], which is an adaptive process for maintaining cell viability [46]. In brief, accumulation of unfolded/misfolded proteins in the ER lumen causes the ER-resident chaperone GRP78/BIP to dissociate from ER sensor proteins, i.e., protein kinase RNA-like ER kinase (PERK), inositol-requiring protein 1α (IRE1α), and activating transcription factor (ATF) 6. Activated PERK phosphorylates eukaryotic initiation factor (eIF) 2α, inhibiting global protein synthesis but increasing the translation of select mRNAs required for stress alleviation. Consistent with the previous findings [45], we showed that COPA silencing induced the expression of ER stress genes, such as ATF4, total XBP1 (tXBP1), spliced XBP1 (sXBP1) [47–49], C/EBP-homologous protein (CHOP), binding immunoglobulin protein (BIP) [50], and ER degradation enhancing alpha-mannosidase-like protein 1 (EDEM1) [51], in differentiated keratinocytes (Fig. 7e). Moreover, we found that HOXC13-AS KD reduced ER stress gene expression in calcium-induced differentiated keratinocytes, whereas HOXC13-AS OE increased ER stress gene expression in these cells (Fig. 7f, g). Similarly, a western blot analysis of a panel of well-established ER stress indicators, including PERK, IRE1α, and BIP, protein disulfide isomerase (PDI), and calnexin [46, 52, 53], revealed that HOXC13-AS KD inhibited and HOXC13-AS OE promoted the accumulation of these ER stress proteins in suspension-induced differentiated keratinocytes (Fig. 7h). Also, in organotypic human epidermal tissues, HOXC13-AS OE enhanced the expression of ER stress marker PDI, as shown by IF staining (Supplementary Fig. 5b). Furthermore, we showed that silencing HOXC13-AS increased general protein synthesis in differentiated keratinocytes induced by calcium, likely due to the alleviated ER stress (Fig. 7i). In line with this, western blot analysis unraveled that HOXC13-AS KD decreased the phosphorylation of PERK and eIF2α (Fig. 7j). Interestingly, treating cells with PERK or eIF2α inhibitors reduced KRT10 expression, which confirmed the role of UPR in keratinocyte differentiation [54] (Fig. 7j). However, only blocking eIF2α, but not PERK, revoked the impact of HOXC13-AS depletion on KRT10 expression, suggesting that PERK activation is not essential for the pro-differentiation effect of HOXC13-AS and other integrated stress response pathways that converge on eIF2α phosphorylation may be also involved [55] (Fig. 7j).
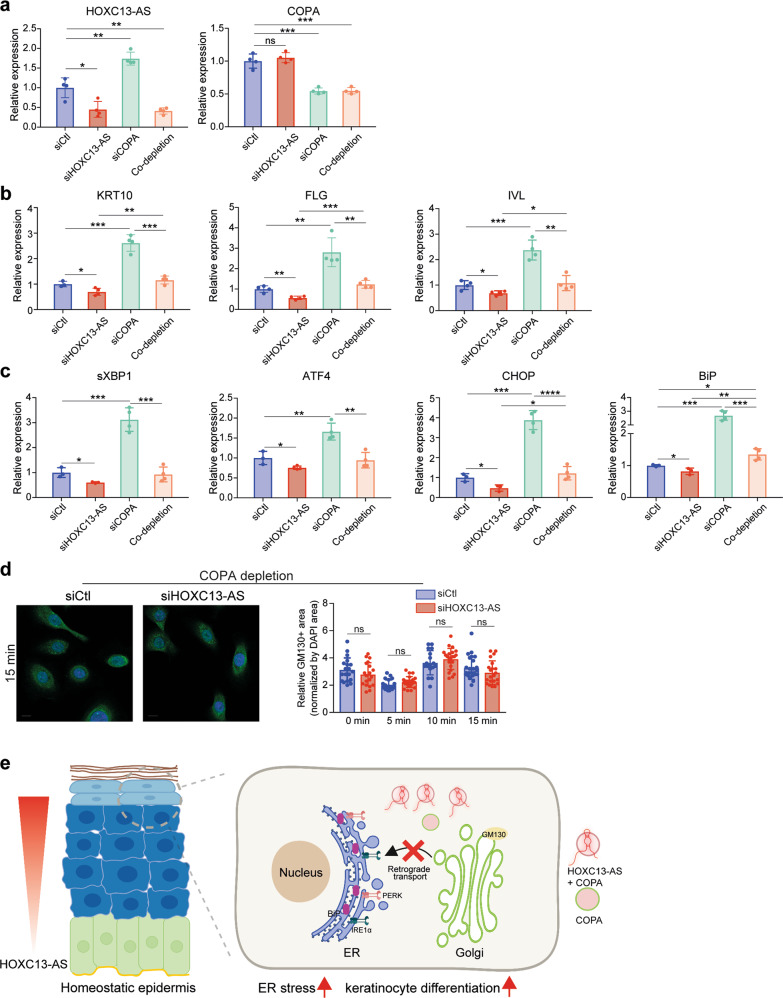
Physiological ER stress has been known to play important roles in keratinocyte differentiation [56]. In line with previous studies [30, 57–59], we showed that the expression of ER stress markers PDI and XBP1 was most enriched in the granular layer keratinocytes of human skin, suggesting that UPR is activated during epidermal differentiation (Fig. 7k, l). Additionally, pharmacological ER stressors, e.g., tunicamycin, have been shown to stimulate the expression of differentiation-related genes [30, 59]. Here, we found that tunicamycin treatment also elevated HOXC13-AS expression in keratinocytes (Supplementary Fig. 7d). Furthermore, we showed that HOXC13-AS level was enhanced by COPA silencing that promoted ER stress (Supplementary Fig. 7e). Importantly, we found that co-silencing COPA with HOXC13-AS rescued the repressive effects of HOXC13-AS KD on keratinocyte differentiation, ER stress, and GM130 retrograde transport, confirming the essential role of COPA in mediating HOXC13-AS’s biological functions (Fig. 8a–d). This constellation of findings suggests that by trapping COPA proteins, HOXC13-AS hampers Golgi–ER retrograde transport and leads to ER stress, thus promoting keratinocyte differentiation (Fig. 8e).
Fig. 8. COPA is important in mediating the biological functions of HOXC13-AS.
QRT-PCR analysis of HOXC13-AS and COPA (a), differentiation markers (b), or ER stress markers (c) in differentiated keratinocytes with individual HOXC13-AS or COPA KD, or co-depletion of HOXC13-AS and COPA (n = 3–4). d Representative photograph and quantification of immunofluorescence staining of GM130 in keratinocytes with COPA and HOXC13-AS KD, and then treated with Brefeldin A (n = 17–25). Cell nuclei were co-stained with DAPI. Scale bar = 10 μm. e A proposed model of HOXC13-AS/COPA-mediated regulation of keratinocyte differentiation. ns not significant, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 by unpaired two-tailed Student’s t test (a–d). Data are presented as mean ± SD.
Discussion
The search for gene expression regulatory mechanisms driving keratinocyte state switching between homeostasis and regeneration led to our identification of HOXC13-AS, a recently evolved, nonconserved human lncRNA. The epidermal-specific expression of HOXC13-AS raised the possibility that it may have evolved to regulate epidermis-related functions. As shown in our study, HOXC13-AS plays a crucial role in promoting keratinocyte differentiation, leading to the establishment of an effective epidermal barrier. To the best of our knowledge, this is the first report showing the physiological role played by HOXC13-AS. This lncRNA has been previously identified as a cancer biomarker in cervical cancer [60], breast cancer [61], glioma [62], hepatocellular carcinoma [63], cholangiocarcinoma [64], and head and neck squamous cell carcinoma [65–67], which emphasizes the absence of HOXC13-AS expression in most normal tissues except the skin. Additionally, HOXC13-AS reportedly functions as an oncogene promoting proliferation and the epithelial-mesenchymal transition of cancer cells [65]. However, we did not observe a significant effect on normal keratinocyte migration or proliferation (Supplementary Fig. 4a–c). Importantly, our study revealed the highly specific expression and functional pattern of HOXC13-AS in human skin, which is a crucial feature endowing lncRNAs with promising therapeutic and diagnostic potential [10–12].
Our study adds HOXC13-AS to the short list of lncRNAs known to modulate epidermal cell differentiation [13–16], which includes both negative (ANCR and LINC00941) and positive regulators (TINCR, uc.291, and PRANCR) [13–17]. Among these lncRNAs, only TINCR, similar to HOXC13-AS, was downregulated in human wound tissues and wound-edge keratinocytes compared to the skin in our RNA-seq analysis (Fig. 1b, c). Prior studies have shown that TINCR directly binds to the STAU1 protein, thus stabilizing differentiation-related mRNAs [14]. In addition to acting as a lncRNA, TINCR encodes a protein named TINCR-encoded ubiquitin-like protein (TUBL), which promotes keratinocyte proliferation and wound repair [68, 69]. Further study to determine whether TINCR may play a role in keratinocyte homeostasis-to-regeneration state transition is warranted, and if TINCR is found to be involved, the determination of whether it functions as a lncRNA or TUBL in this process will be needed.
We discovered that HOXC13-AS acts via a unique mechanism, i.e., sequestration of COPA, a protein required for retrograde Golgi-to-ER transport to recycle the ER-derived transport machinery and resident proteins [70, 71]. Mutant COPA has been previously shown to impair the assembly of proteins targeted for transport and to lead to ER stress and UPR activation in hereditary autoimmune-mediated lung disease and arthritis [41, 45]. Previous studies have demonstrated that physiological ER stress is required to modulate keratinocyte differentiation [56]. Our study not only confirmed the importance of COPA in maintaining ER homeostasis but also uncovered its inhibitory role on keratinocyte differentiation. We revealed a novel regulatory mechanism for COPA-mediated Golgi-to-ER retrograde transport, i.e., the lncRNA HOXC13-AS traps COPA protein, thus hindering COPA-mediated cargo assembly, which results in ER stress and promotes keratinocyte differentiation (Fig. 8e). In contrast to the physiological levels of ER stress that are required for modulation of keratinocyte differentiation, persistent or excessive levels of ER stress lead to cell death and apoptosis, which has been detected in skin diseases with aberrant epidermal differentiation, such as Darier’s disease [72]. It is tempting to explore whether HOXC13-AS may play a pathological role or even serve as a therapeutic target in human diseases with chronic ER stress.
During human skin wound healing, the expression of HOXC13-AS in wound-edge keratinocytes was transiently downregulated, likely due to high EGFR signaling in the wound environment. It has been previously shown that sustained activation of EGFR signaling suppressed keratinocyte differentiation, whereas its blockade induced differentiation through the activation of Notch signaling [29]. In this study, we added an additional mechanistic link between EGFR signaling and epidermal differentiation, i.e., EGFR signaling inhibited the expression of HOXC13-AS, a crucial positive regulator of keratinocyte differentiation. In addition to its differentiation-promoting function, HOXC13-AS suppressed the innate immune response in keratinocytes; thus, its rapid downregulation upon skin injury may facilitate the initiation of the inflammatory stage of wound repair (Supplementary Fig. 3k). Moreover, in homeostatic skin, the enrichment of HOXC13-AS in differentiated keratinocytes, which comprise the outermost layers of the epidermis, may contribute to the maintenance of immune tolerance of the skin barrier, which is constantly exposed to the harsh external environment.
Taken together, the data obtained through our study shows that a human-specific lncRNA, HOXC13-AS, is a crucial regulator of epidermal differentiation and that it functions by sequestrating the COPA protein and interfering with Golgi-to-ER transport. These findings contribute to understanding the molecular mechanisms required to maintain and regenerate the epidermal barrier. The specific expression in human skin and the critical function in regulating ER stress make HOXC13-AS a potential therapeutic target for a range of cutaneous diseases characterized by chronic ER stress.
Supplementary information
Acknowledgements
We express our gratitude to all the tissue donors participating in this study. We thank the Microarray core facility at Novum, BEA, which is supported by the board of research at Karolinska Institute and the research committee at the Karolinska hospital. In-gel digestion, peptide extraction, mass spectrometric analysis, and database searches for protein identification were carried out at the Proteomics Biomedicum, Karolinska Institute, Stockholm. The computations/data handling was enabled by resources in the projects sens2020010 and 2021/22-701 provided by the Swedish National Infrastructure for Computing (SNIC) at UPPMAX, partially funded by the Swedish Research Council through grant agreement no. 2018-05973.
Author contributions
NXL and LZ conceived and designed the study. PS collected most clinical samples with the assistance of MAT and XB. LZ, MP, DL, XB, GN, and JG performed the experiments. ZL and LZ carried out bioinformatics analysis. LZ, MP, and NXL contributed to data analyses and interpretation. LZ, MP, and NXL wrote the manuscript, which was commented on by all the authors.
Funding
This work was supported by Swedish Research Council (Vetenskapsradet, 2020-01400), Ragnar Söderbergs Foundation (M31/15), Welander and Finsens Foundation (Hudfonden), LEO foundation, Cancerfonden, Ming Wai Lau Centre for Reparative Medicine, and Karolinska Institute. Open access funding provided by Karolinska Institute.
Data availability
Raw data of long RNA-sequencing and microarray performed for this study have been deposited to NCBI’s Gene Expression Omnibus (GEO) database under the accession numbers GSE174661 and GSE206103, respectively.
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Stockholm Regional Ethics Committee (Stockholm, Sweden) and conducted according to the Declaration of Helsinki’s principles.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41418-023-01142-z.
References
- 1.Cañedo-Dorantes L, Cañedo-Ayala M. Skin acute wound healing: a comprehensive review. Int J Inflamm. 2019;2019:3706315. doi: 10.1155/2019/3706315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goleva E, Berdyshev E, Leung DY. Epithelial barrier repair and prevention of allergy. J Clin Invest. 2019;129:1463–74. doi: 10.1172/JCI124608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–40. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 4.Wikramanayake TC, Stojadinovic O, Tomic-Canic M. Epidermal differentiation in barrier maintenance and wound healing. Adv Wound Care. 2014;3:272–80. doi: 10.1089/wound.2013.0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinengo L, Olsson M, Bajpai R, Soljak M, Upton Z, Schmidtchen A, et al. Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Ann Epidemiol. 2019;29:8–15. doi: 10.1016/j.annepidem.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Sen CK. Human wounds and its burden: an updated compendium of estimates. Adv Wound Care. 2019;8:39–48. doi: 10.1089/wound.2019.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartel DP. Metazoan microRNAs. Cell. 2018;173:20–51. doi: 10.1016/j.cell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hon CC, Ramilowski JA, Harshbarger J, Bertin N, Rackham OJ, Gough J, et al. An atlas of human long non-coding RNAs with accurate 5’ ends. Nature. 2017;543:199–204. doi: 10.1038/nature21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gloss BS, Dinger ME. The specificity of long noncoding RNA expression. Biochim Biophys Acta. 2016;1859:16–22. doi: 10.1016/j.bbagrm.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Liu SJ, Horlbeck MA, Cho SW, Birk HS, Malatesta M, He D, et al. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science. 2017;355:aah7111. [DOI] [PMC free article] [PubMed]
- 12.Winkle M, El-Daly SM, Fabbri M, Calin GA. Noncoding RNA therapeutics—challenges and potential solutions. Nat Rev Drug Discov. 2021;20:629–51. doi: 10.1038/s41573-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kretz M, Webster DE, Flockhart RJ, Lee CS, Zehnder A, Lopez-Pajares V, et al. Suppression of progenitor differentiation requires the long noncoding RNA ANCR. Genes Dev. 2012;26:338–43. doi: 10.1101/gad.182121.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kretz M, Siprashvili Z, Chu C, Webster DE, Zehnder A, Qu K, et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature. 2013;493:231–5. doi: 10.1038/nature11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziegler C, Graf J, Faderl S, Schedlbauer J, Strieder N, Forstl B, et al. The long non-coding RNA LINC00941 and SPRR5 are novel regulators of human epidermal homeostasis. EMBO Rep. 2019;20:e46612. [DOI] [PMC free article] [PubMed]
- 16.Panatta E, Lena AM, Mancini M, Smirnov A, Marini A, Delli Ponti R, et al. Long non-coding RNA uc.291 controls epithelial differentiation by interfering with the ACTL6A/BAF complex. EMBO Rep. 2020;21:e46734. doi: 10.15252/embr.201846734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai P, Otten ABC, Cheng B, Ishii MA, Zhang W, Huang B, et al. A genome-wide long noncoding RNA CRISPRi screen identifies PRANCR as a novel regulator of epidermal homeostasis. Genome Res. 2020;30:22–34. doi: 10.1101/gr.251561.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li D, Kular L, Vij M, Herter EK, Li X, Wang A, et al. Human skin long noncoding RNA WAKMAR1 regulates wound healing by enhancing keratinocyte migration. Proc Natl Acad Sci USA. 2019;116:9443–52.. doi: 10.1073/pnas.1814097116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herter EK, Li D, Toma MA, Vij M, Li X, Visscher D, et al. WAKMAR2, a long noncoding RNA downregulated in human chronic wounds, modulates keratinocyte motility and production of inflammatory chemokines. J Invest Dermatol. 2019;139:1373–84. doi: 10.1016/j.jid.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 20.Li D, Niu G, Landen NX. Beyond the code: noncoding RNAs in skin wound healing. Cold Spring Harb Perspect Biol. 2022;14:1–28. [DOI] [PMC free article] [PubMed]
- 21.Everaert C, Volders PJ, Morlion A, Thas O, Mestdagh P. SPECS: a non-parametric method to identify tissue-specific molecular features for unbalanced sample groups. BMC Bioinforma. 2020;21:58. doi: 10.1186/s12859-020-3407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Consortium GT. The genotype-tissue expression (GTEx) project. Nat Genet. 2013;45:580–5. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang YJ, Yang DC, Kong L, Hou M, Meng YQ, Wei L, et al. CPC2: a fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017;45:W12–6. doi: 10.1093/nar/gkx428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajjari M, Salavaty A. HOTAIR: an oncogenic long non-coding RNA in different cancers. Cancer Biol Med. 2015;12:1–9. doi: 10.7497/j.issn.2095-3941.2015.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawaya AP, Stone RC, Brooks SR, Pastar I, Jozic I, Hasneen K, et al. Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nat Commun. 2020;11:4678. doi: 10.1038/s41467-020-18276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iglesias-Bartolome R, Uchiyama A, Molinolo AA, Abusleme L, Brooks SR, Callejas-Valera JL, et al. Transcriptional signature primes human oral mucosa for rapid wound healing. Sci Transl Med. 2018;10:eaap8798. [DOI] [PMC free article] [PubMed]
- 27.Liu Z, Zhang L, Toma MA, Li D, Bian X, Pastar I, et al. Integrative small and long RNA omics analysis of human healing and nonhealing wounds discovers cooperating microRNAs as therapeutic targets. Elife. 2022;11:e80322. [DOI] [PMC free article] [PubMed]
- 28.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 29.Kolev V, Mandinova A, Guinea-Viniegra J, Hu B, Lefort K, Lambertini C, et al. EGFR signalling as a negative regulator of Notch1 gene transcription and function in proliferating keratinocytes and cancer. Nat Cell Biol. 2008;10:902–11. doi: 10.1038/ncb1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugiura K, Muro Y, Futamura K, Matsumoto K, Hashimoto N, Nishizawa Y, et al. The unfolded protein response is activated in differentiating epidermal keratinocytes. J Invest Dermatol. 2009;129:2126–35. doi: 10.1038/jid.2009.51. [DOI] [PubMed] [Google Scholar]
- 31.Bikle DD, Xie Z, Tu CL. Calcium regulation of keratinocyte differentiation. Expert Rev Endocrinol Metab. 2012;7:461–72. doi: 10.1586/eem.12.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elsholz F, Harteneck C, Muller W, Friedland K. Calcium-a central regulator of keratinocyte differentiation in health and disease. Eur J Dermatol. 2014;24:650–61. doi: 10.1684/ejd.2014.2452. [DOI] [PubMed] [Google Scholar]
- 33.Kouwenhoven EN, Oti M, Niehues H, van Heeringen SJ, Schalkwijk J, Stunnenberg HG, et al. Transcription factor p63 bookmarks and regulates dynamic enhancers during epidermal differentiation. EMBO Rep. 2015;16:863–78. doi: 10.15252/embr.201439941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chidgey M, Brakebusch C, Gustafsson E, Cruchley A, Hail C, Kirk S, et al. Mice lacking desmocollin 1 show epidermal fragility accompanied by barrier defects and abnormal differentiation. J Cell Biol. 2001;155:821–32. doi: 10.1083/jcb.200105009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denecker G, Hoste E, Gilbert B, Hochepied T, Ovaere P, Lippens S, et al. Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol. 2007;9:666–74. doi: 10.1038/ncb1597. [DOI] [PubMed] [Google Scholar]
- 36.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vietri Rudan M, Mishra A, Klose C, Eggert US, Watt FM. Human epidermal stem cell differentiation is modulated by specific lipid subspecies. Proc Natl Acad Sci USA. 2020;117:22173–82. doi: 10.1073/pnas.2011310117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Q, Kim SH, Perez-Lorenzo R, Liu C, Huang M, Dotto GP, et al. Phenformin promotes keratinocyte differentiation via the calcineurin/NFAT pathway. J Invest Dermatol. 2021;141:152–63. doi: 10.1016/j.jid.2020.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bridges MC, Daulagala AC, Kourtidis A. LNCcation: lncRNA localization and function. J Cell Biol. 2021;220:e202009045. [DOI] [PMC free article] [PubMed]
- 40.Panda AC, Martindale JL, Gorospe M. Affinity pulldown of biotinylated RNA for detection of protein-RNA complexes. Bio Protoc. 2016;6:e2062. [DOI] [PMC free article] [PubMed]
- 41.Lepelley A, Martin-Niclos MJ, Le Bihan M, Marsh JA, Uggenti C, Rice GI, et al. Mutations in COPA lead to abnormal trafficking of STING to the Golgi and interferon signaling. J Exp Med. 2020;217:e20200600. [DOI] [PMC free article] [PubMed]
- 42.Citterio C, Vichi A, Pacheco-Rodriguez G, Aponte AM, Moss J, Vaughan M. Unfolded protein response and cell death after depletion of brefeldin A-inhibited guanine nucleotide-exchange protein GBF1. Proc Natl Acad Sci USA. 2008;105:2877–82. doi: 10.1073/pnas.0712224105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ivessa NE, De Lemos-Chiarandini C, Gravotta D, Sabatini DD, Kreibich G. The Brefeldin A-induced retrograde transport from the Golgi apparatus to the endoplasmic reticulum depends on calcium sequestered to intracellular stores. J Biol Chem. 1995;270:25960–7. doi: 10.1074/jbc.270.43.25960. [DOI] [PubMed] [Google Scholar]
- 44.Lubbehusen J, Thiel C, Rind N, Ungar D, Prinsen BH, de Koning TJ, et al. Fatal outcome due to deficiency of subunit 6 of the conserved oligomeric Golgi complex leading to a new type of congenital disorders of glycosylation. Hum Mol Genet. 2010;19:3623–33. doi: 10.1093/hmg/ddq278. [DOI] [PubMed] [Google Scholar]
- 45.Watkin LB, Jessen B, Wiszniewski W, Vece TJ, Jan M, Sha Y, et al. COPA mutations impair ER-Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis. Nat Genet. 2015;47:654–60. doi: 10.1038/ng.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 47.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–6. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 48.Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, et al. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002;16:452–66. doi: 10.1101/gad.964702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–91. doi: 10.1016/S0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 50.Sato N, Urano F, Yoon Leem J, Kim SH, Li M, Donoviel D, et al. Upregulation of BiP and CHOP by the unfolded-protein response is independent of presenilin expression. Nat Cell Biol. 2000;2:863–70. doi: 10.1038/35046500. [DOI] [PubMed] [Google Scholar]
- 51.Olivari S, Cali T, Salo KE, Paganetti P, Ruddock LW, Molinari M. EDEM1 regulates ER-associated degradation by accelerating de-mannosylation of folding-defective polypeptides and by inhibiting their covalent aggregation. Biochem Biophys Res Commun. 2006;349:1278–84. doi: 10.1016/j.bbrc.2006.08.186. [DOI] [PubMed] [Google Scholar]
- 52.Bergeron JJ, Brenner MB, Thomas DY, Williams DB. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem Sci. 1994;19:124–8. doi: 10.1016/0968-0004(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 53.Williams DB. Beyond lectins: the calnexin/calreticulin chaperone system of the endoplasmic reticulum. J Cell Sci. 2006;119:615–23. doi: 10.1242/jcs.02856. [DOI] [PubMed] [Google Scholar]
- 54.Collier AE, Wek RC, Spandau DF. Human keratinocyte differentiation requires translational control by the eIF2alpha kinase GCN2. J Invest Dermatol. 2017;137:1924–34. doi: 10.1016/j.jid.2017.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM. The integrated stress response. EMBO Rep. 2016;17:1374–95. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park K, Lee SE, Shin KO, Uchida Y. Insights into the role of endoplasmic reticulum stress in skin function and associated diseases. FEBS J. 2019;286:413–25. doi: 10.1111/febs.14739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park K, Elias PM, Oda Y, Mackenzie D, Mauro T, Holleran WM, et al. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin D receptor-independent pathway. J Biol Chem. 2011;286:34121–30. doi: 10.1074/jbc.M111.250431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Celli A, Sanchez S, Behne M, Hazlett T, Gratton E, Mauro T. The epidermal Ca(2+) gradient: Measurement using the phasor representation of fluorescent lifetime imaging. Biophys J. 2010;98:911–21. doi: 10.1016/j.bpj.2009.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Celli A, Mackenzie DS, Crumrine DS, Tu CL, Hupe M, Bikle DD, et al. Endoplasmic reticulum Ca2+ depletion activates XBP1 and controls terminal differentiation in keratinocytes and epidermis. Br J Dermatol. 2011;164:16–25. doi: 10.1111/j.1365-2133.2010.10046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang T, Li W, Ye B, Zhang S, Lei X, Zhang D. FTO-stabilized lncRNA HOXC13-AS epigenetically upregulated FZD6 and activated Wnt/beta-catenin signaling to drive cervical cancer proliferation, invasion, and EMT. J BUON. 2021;26:1279–91. [PubMed] [Google Scholar]
- 61.Li X, Wang Q, Rui Y, Zhang C, Wang W, Gu J, et al. HOXC13-AS promotes breast cancer cell growth through regulating miR-497-5p/PTEN axis. J Cell Physiol. 2019;234:22343–51. doi: 10.1002/jcp.28800. [DOI] [PubMed] [Google Scholar]
- 62.Liu N, Wang Z, Liu D, Xie P. HOXC13-AS-miR-122-5p-SATB1-C-Myc feedback loop promotes migration, invasion and EMT process in glioma. OncoTargets Ther. 2019;12:7165–73. doi: 10.2147/OTT.S220027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou JF, Shi YT, Wang HG, Yang XZ, Wu SN. Overexpression of long noncoding RNA HOXC13-AS and its prognostic significance in hepatocellular carcinoma. Eur Rev Med Pharm Sci. 2019;23:7369–74. doi: 10.26355/eurrev_201909_18843. [DOI] [PubMed] [Google Scholar]
- 64.Angenard G, Merdrignac A, Louis C, Edeline J, Coulouarn C. Expression of long non-coding RNA ANRIL predicts a poor prognosis in intrahepatic cholangiocarcinoma. Dig Liver Dis. 2019;51:1337–43. doi: 10.1016/j.dld.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 65.Li W, Zhu Q, Zhang S, Liu L, Zhang H, Zhu D. HOXC13-AS accelerates cell proliferation and migration in oral squamous cell carcinoma via miR-378g/HOXC13 axis. Oral Oncol. 2020;111:104946. doi: 10.1016/j.oraloncology.2020.104946. [DOI] [PubMed] [Google Scholar]
- 66.Xiong D, Wu W, Kan L, Chen D, Dou X, Ji X, et al. LINC00958 and HOXC13-AS as key candidate biomarkers in head and neck squamous cell carcinoma by integrated bioinformatics analysis. PeerJ. 2020;8:e8557. doi: 10.7717/peerj.8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao C, Lu W, Lou W, Wang L, Xu Q. Long noncoding RNA HOXC13-AS positively affects cell proliferation and invasion in nasopharyngeal carcinoma via modulating miR-383-3p/HMGA2 axis. J Cell Physiol. 2019;234:12809–20. doi: 10.1002/jcp.27915. [DOI] [PubMed] [Google Scholar]
- 68.Nita A, Matsumoto A, Tang R, Shiraishi C, Ichihara K, Saito D, et al. A ubiquitin-like protein encoded by the “noncoding” RNA TINCR promotes keratinocyte proliferation and wound healing. PLoS Genet. 2021;17:e1009686. doi: 10.1371/journal.pgen.1009686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eckhart L, Lachner J, Tschachler E, Rice RH. TINCR is not a non-coding RNA but encodes a protein component of cornified epidermal keratinocytes. Exp Dermatol. 2020;29:376–9. doi: 10.1111/exd.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burri L, Varlamov O, Doege CA, Hofmann K, Beilharz T, Rothman JE, et al. A SNARE required for retrograde transport to the endoplasmic reticulum. Proc Natl Acad Sci USA. 2003;100:9873–7. doi: 10.1073/pnas.1734000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mukai K, Ogawa E, Uematsu R, Kuchitsu Y, Kiku F, Uemura T, et al. Homeostatic regulation of STING by retrograde membrane traffic to the ER. Nat Commun. 2021;12:61. doi: 10.1038/s41467-020-20234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sakuntabhai A, Ruiz-Perez V, Carter S, Jacobsen N, Burge S, Monk S, et al. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet. 1999;21:271–7. doi: 10.1038/6784. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data of long RNA-sequencing and microarray performed for this study have been deposited to NCBI’s Gene Expression Omnibus (GEO) database under the accession numbers GSE174661 and GSE206103, respectively.