Abstract
In traditional medicine, Tarooneh (a hardcover of the date palm; Phoenix dactylifera) has known as a sedative and relaxant medicine. In this study, we evaluated the protective effects of Tarooneh in the anxiety-like behavior, cognitive deficit, and neuronal damages in the CA1, CA3, and dentate gyrus (DG) regions of the hippocampus and frontal cortex neurons employing a rat model of chronic restraint stress. The animal received Tarooneh extract for 14 consecutive days in water, and chronic restraint stress was performed daily during this period. The results of the Barnes maze test showed that treatment with Tarooneh significantly improves spatial memory parameters such as latency time to find the target hole, number of errors, and distance traveling compared to the stress group. The EPM results showed that Tarooneh significantly increased the time spent in open arms and the percentage of entries into open arms and significantly decreased the frequency of head dipping behavior compared to animals in the stress group. Golgi–Cox staining indicates that loss of neural spine density in DG, CA1, CA3, and frontal cortex due to chronic restraint stress, was prevented with daily administration of Tarooneh. The results of cresyl-violet staining indicate that Tarooneh significantly increased the number of CV-positive neurons in the frontal cortex and CA1 region of the hippocampus compared to the stress group. Our results suggest that Tarooneh potentially prevented and improved effects in anxiety-like behavior, memory impairment, and synaptic plasticity loss in frontal and hippocampal neurons induced by chronic restraint stress. In conclusion, our results suggest that Tarooneh prevented and improved anxiety-like behavior, cognitive deficit, and neuronal damages in the CA1, CA3, and DG regions of the hippocampus and frontal cortex neurons induced by chronic restraint stress.
Keywords: Tarooneh, Chronic restraint stress, Anxiety, Memory impairment, Neural plasticity
Introduction
Extended exposure to stress may lead to different neuropsychiatric disorders. There is a close relationship between chronic stress and anxiety disorders (Chrousos 2009; Dagnino-Subiabre 2021; Speers et al. 2021). Various studies have reported that stressful stimuli lead to impaired synaptic plasticity in the hippocampus and inhibition of neurogenesis (Kim et al. 2013; Maggio et al. 2013; Schoenfeld and Gould 2013; Price and Duman 2020; Garcia-Keller et al. 2021). In addition, epidemiological studies show that the risk of dementia and exacerbation of neurological disorders, such as Alzheimer's disease or major depression, is associated with stress (Wilson et al. 2007; Najjar et al. 2013; Escher et al. 2019; Srivastava et al. 2019; Tripathi et al. 2019; Saeedi and Rashidy-Pour 2021, de Sousa et al. 2021).
The effect of stress depends on the type of stress, the area of the brain affected, and the individual sensitivity (Chen et al. 2012; Joëls et al. 2018). Experimental animal models should confirm the pathobiology features of human diseases. One commonly used stress model is the restraint model, which is a modified model of immobility stress. In this method, where physical and mental stress is unavoidable, the animals are placed in a plastic tube to prevent movement. This stress model is valid and has physical and psychological effects (Jaggi et al. 2011; Son et al. 2019). The frontal cortex and hippocampus are the brain's most sensitive parts to stress (Kim and Diamond 2002; Radley et al. 2006; Mograbi et al. 2020). Chronic restraint stress leads to learning and memory impairment and neuronal damage to the frontal cortex and the hippocampus (Huang et al. 2015; Mograbi et al. 2020). The expression of the β-actin protein gene, a significant component of dendritic spines and amyloid precursor protein and has been linked to Alzheimer's disease in the hippocampus, is altered by immobility stress (Rai et al. 2020a, b; Sántha et al. 2012).
Phoenix dactylifera (date palm) is a diploid plant of the monocotyledonous family Aceraceae, which is often cultivated in Middle East countries and is well known in these areas, because different parts of the plant have long used it for food and household appliances (Rahimi et al. 2015). In traditional medicine, different palm tree parts have been used to treat various disorders. Plant extracts were common in traditional medicine and had a high status up to 50 years ago (Hosseini 2018). Therefore, paying attention to herbal medicines is very important in producing new medicines and can establish a good relationship between traditional and modern medicine.
Tarooneh is a hardcover of date pollen that opens during the pollination season and remains like a shell. Tarooneh extract has been a sedative and soothing substance since ancient times. Various studies have also examined the properties of Tarooneh. It has been shown that Tarooneh relieves headaches and diarrhea (Al-Taher 2008), also effects on LH (luteinizing hormone), FSH (follicle stimulating hormone), and testosterone concentrations (Mokhtari and Sharifi 2007), reduction of chronic pain as well as therapeutic properties of hypolipidemia, increased milk in lactating mothers, sedative and nerve relaxing properties, joint pain reliever, and treatment of rheumatic diseases have been reported (Rahimi et al. 2017).
According to these studies, Tarooneh may have neuroprotective effects; however, the underlying mechanism of neuroprotective effects of Tarooneh is far from clear. On the other hand, though some studies evaluated the neuroprotective effects of the hydroalcoholic extract of Tarooneh in rodents (Rahimi et al. 2015), according to our knowledge, there is no study conducted on the effects of Tarooneh on spine density in the CA1, CA3, and dentate gyrus (DG) regions of the hippocampus and frontal cortex neurons in chronic immobilization stress. Thus, this study aimed to investigate the effect of Tarooneh on anxiety and cognitive deficit, as well as its effect on spine density and neuronal damages in the frontal cortex and hippocampus CA1, CA3, and DG regions neurons on chronic immobilization stress in rats.
Materials and methods
Animals
Adult male Wistar rats weighing 200–220 g were obtained from the Baqiyatallah University of Medical Science animal house (Tehran, IR. Iran). The rats were housed in a polycarbonate rodent cage under standard laboratory conditions with a 12 h light/dark cycle at constant temperature (22 ± 1 °C) and humidity (55 ± 5%) in the conventional animal house of the Baqiyatallah Neuroscience Research Centre, Tehran, Iran. Animals have access to drinking water and a standard chow diet ad libitum. At least 7 days after the animals arrived, the animal experiments were begun. The experiments were conducted in accordance with the animal care and use guidelines approved by the local ethical committee (The Baqiyatallah University of Medical Committee on the Use and Care of Animals; Protocol approval number: IR.BMSU.REC.1400.089).
Plant and extraction
A pharmacological maceration technique was used to extract the spathe of phoenix dactylifera that were gathered from the South of the Fars province of Iran. The gathered pieces were cleaned and dried at room temperature away from direct sunlight. Then, 70% alcohol was added to the dried and ground spathe powder, using 400 ml of alcohol for every 100 g of powder. The valves containing the solvent were sealed to prevent alcohol evaporation. The solvent was then condensed by rotary at 40 °C and in a bain-marie (water bath) for approximately 6 h, followed by freeze-drying of the supernatant (Rahimi et al. 2019).
Experimental groups
The rats were randomly divided into four groups of six individuals each, as follows:
The unstressed group daily received tap water instead of Tarooneh (Control group, n = 6).
Restraint-stressed group daily received tap water instead of Tarooneh (Stress group, n = 6).
The unstressed group received Tarooneh extract 125 mg/kg/day (Tarooneh group, n = 6).
The restraint-stressed plus Tarooneh-treated group received Tarooneh extract 125 mg/kg/day (Stress + Tarooneh group, n = 6).
The procedure of restraint stress was carried out for 14 consecutive days once daily for 2 h from 09:00 a.m. to 11:00 a.m. in rodent restraint tubes. In brief, rats were placed in plastic tubes (20 × 9 × 7 cm), of which one end is conical and has several 3-mm holes for breathing, and the other is open. The animals could not move within the tubes but could breathe normally (Lee et al. 2013).
Behavioral tests
Elevated Plus Maze (EPM)
Elevated Plus Maze (EPM) was used to evaluate the anxiety-like behavior (Roohbakhsh et al. 2007). The apparatus consists of two opposing closed and two open arms (50 cm × 10 cm) with 50 cm-high walls of the exact dimensions of closed arms and a central platform (10 cm × 10 cm) at the intersection of the open and closed arms. The maze was 120 cm raised from the floor. The animal was placed on the central platform at the start, and the task was performed for 5 min. The camera placed above the maze recorded the animal's behaviors during the test. The analyzed parameters include time spent in the open arms and the number of open arms entries, head dipping behavior frequency.
Barnes maze
The Barnes maze is a strongly approved test to estimate cognitive impairment in learning and memory in animals (Hadipour et al. 2018). The maze is elevated 1 m above the floor, and an overhead light source (500 W, 1000 lx) shines down on the table surface. All animals were adapted to the maze before beginning the trials. Barnes maze included 4 days of the training task, including four times each 90 s trials and a single 90 s probe test on day 5. During training trials, rats were put in the maze's center and allowed up to 90 s to find the escape box. The time to find the hidden box was recorded as escape latency time; the number of errors and distance taken to reach the box were recorded. If, in training days, an animal cannot find the hidden box within 90 s, he is gently directed to the location of the box by the researcher. When an animal entered the escape box, the box was covered for 30 s for the habituation of the animal. The animals were trained four times a day for four continuous training days. On day 5, probe test day, the escape latency time, the number of errors, and the distance were recorded and analyzed.
Histological tests
Golgi–Cox staining
Golgi–Cox staining was used to examine neuronal arborization (Zaqout and Kaindl 2016). The Golgi–Cox solution was prepared from 5% potassium dichromate, 5% mercuric chloride, and potassium chromate in a 5:5:4 vol parts ratio and filtered through filter paper. The solution was mixed in a glass bottle and kept in the dark for at least 48 h before being used to precipitate the formation. The developing solutions included ammonia solution (3:1 ammonia:dd-H2O), 5% Sodium thiosulfate, different concentrations of ethanol (50%, 70%, 90%, and 100%) and xylene. For each brain sample, 10 ml of the upper part of the Golgi–Cox solution was put into a small bottle, and the samples were incubated at room temperature for 7–10 days in the dark. Then the brains were sliced into 100-μm-thick sections. During the development phase, the slides were placed into dd-H2O two times for 5 min each, and the process proceeded as follows; 5 min in 50 percent ethanol, 8 min in 3:1 ammonia, two times in dd-H2O for 5 min each, 10 min in 5 percent sodium phosphate, two times in dd-H2O for 1 min each. After rinsing, the slices were washed with distilled water and collected on clean, coated gelatin glass microscope slides.
The mounted tissue was dehydrated in successive alcohol baths of 70% (6 min), 95% (6 min), and 2 × 100% (6 min), followed by 2 × 6 min in xylene solution. They were then covered with coverslips and histological glue. After tissue processing, the colored sections were evaluated using the Nikon Eclipse 50i (40× magnification) light microscope connected to a Nikon Ds fi1 camera. The number of spine densities on each neuron per 100 μm was estimated. For each group, three animals were selected, five slides were analyzed, and, of all sections, a minimum of seven neurons was selected on each rat. Then, the images were analyzed with the ImageJ software. The shape of the soma, the presence of dendritic and axonal shafts, and no morphological changes of neurons due to the staining procedure were the main criteria for choosing neurons for analysis.
Cresyl violet (CV)
CV staining was performed to examine neuronal damage in the hippocampus and frontal cortex following 14 consecutive days of restraint stress. 5-µm sections from study groups were loaded on albumin-coated slides and deparaffinized with descending serial ethanol. Cresyl violet 0.1% (w/v) was prepared by dissolving CV acetate powder (Sigma-Aldrich) in distilled water, and glacial acetic acid was added to the solution just before staining and filtering. Sections were immersed for 5 min in CV 0.1% solutions, dehydrated in serial ethanol, and mounted with coverslips and histology glue (Li et al. 2011). Three animals in each group were used for CV staining. We counted ten microscopic fields at least in eight slides of each group, and the number of CV-positive cells was reported as mean ± SEM. The number of CV-positive cells in each region was counted per microscopic field in 40× magnitude at Nikon Eclipse 50i light microscope.
Statistical analysis
Data are shown as the mean ± SEM for eight animals. All data were analyzed using IBM SPSS statistics (version 24). All data were analyzed using one-way ANOVA, followed by the Tukey post hoc test.
Results
In the EPM Tarooneh extract increases the time spent in open arms and the percentage of entries in the stressed animals
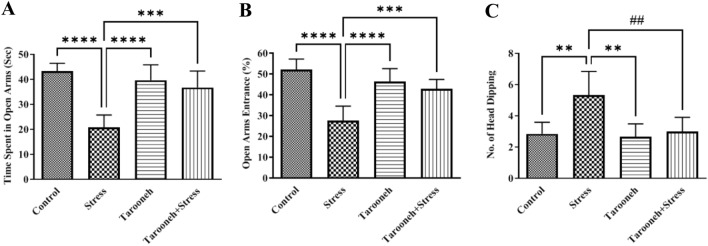
To evaluate anxiety-like behavior in the groups, the animals underwent an EPM on day 14 of the study. Statistical analysis showed that 14 consecutive days of restraint stress significantly decreased the time spent in the open arms (20.80 ± 2.01, F(3,20) = 20.23, P < 0.0001), the percentage of entries into the open arms (27.50 ± 2.86, F(3,20) = 20.02, P < 0.0001), and increased head dipping behavior frequency (5.33 ± 0.61, F(3,20) = 8.82, P = 0.0006), compared to the control group (43.22 ± 1.32, 52.12 ± 2.03, 2.83 ± 0.30, respectively).
The administration of Tarooneh significantly increased the time spent in the open arms (36.72 ± 2.69, P < 0.0001), the percentage of entries into the open arms (42.83 ± 1.84, P < 0.0001), and decreased the frequency of head dipping behavior (3.00 ± 0.36, P = 0.0013) in stressed animals, compared to the stress group (Fig. 1). There were no significant differences between the control, and Tarooneh-treated animals with either of the combined stress + Tarooneh-treated rats.
Fig. 1.
Tarooneh decreased anxiety-like behaviors in the chronic restraint stress in the elevated plus maze. One-way ANOVA revealed significant increases in the time spent in open arms (A), % of open arms entries (B), and the number of head dipping within 5 min in the Tarooneh + stress group compared with the stress group. Values represent the mean ± SEM. **** as P < 0.0001, *** as P < 0.001, ** as P = 0.0006, ## as P = 0.0013
Tarooneh improves latency time, reduces errors, and distance traveled in stressed animals in the Barnes maze
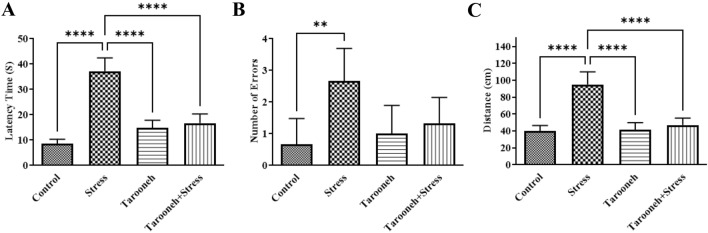
On the tenth day of the study, rats underwent a Barnes maze to evaluate cognitive impairment in learning and memory. As shown in Fig. 2A, the latency time to find the target hole on the fifth day of the task (probe test day) in the stress group significantly increased (37.17 ± 2.16, F(3,20) = 68.43, P < 0.0001) compared to the control group (8.50 ± 0.76). Administering Tarooneh for 14 consecutive days significantly decreased the latency time to achieve the target hole in stressed animals (16.67 ± 1.49). The results showed that the number of errors to find the target hole in the stress group significantly increased (2.66 ± 0.42, F(3,20) = 5.76, P < 0.0052) compared to the control group (0.66 ± 0.33). However, 14 days of administering Tarooneh significantly decreased the number of errors (1.33 ± 0.33) (Fig. 2B). Animals in the stress group had a significant increase in the distance traveled to the target hole (95.17 ± 6.09, F(3,20) = 41.33, P < 0.0001) compared to the control group (39.83 ± 2.73), and Tarooneh decreased the distance traveled (47.17 ± 3.34) compared to the stress group (Fig. 2C). There was no significant difference between the control and Tarooneh groups in Barnes maze tasks (P > 0.05).
Fig. 2.
Effect of Tarooneh extract on chronic restraint stress-induced spatial memory deficits in the Barnes maze task. A Mean latency time to achieve the target hole, B the mean number of errors, and C distance traveling in the probe day (day 5) of training sessions in different groups. Values represent the mean 6 SEM. **** as P < 0.0001, ** as P < 0.0052
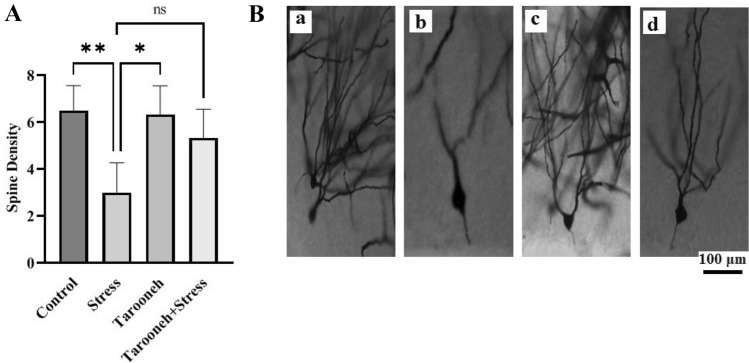
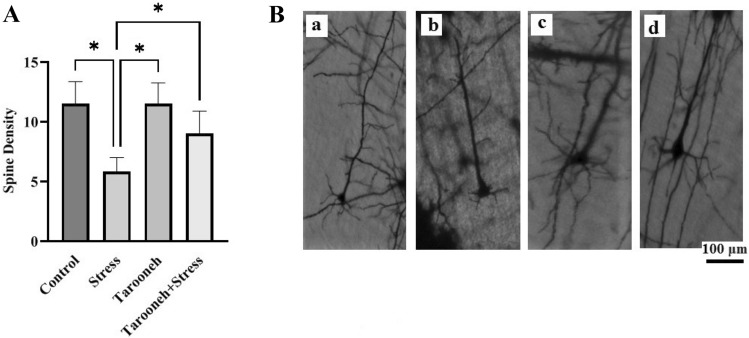
Golgi–Cox showed Tarooneh prevents spine density loss in CA1 neurons and increases spine density in frontal neurons and CA3 and DG regions of the hippocampus in the stressed animals
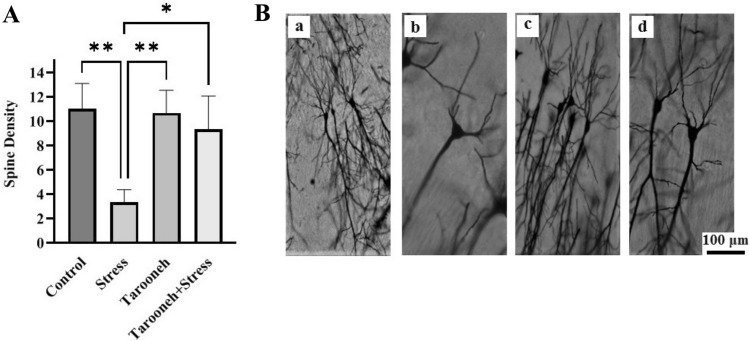
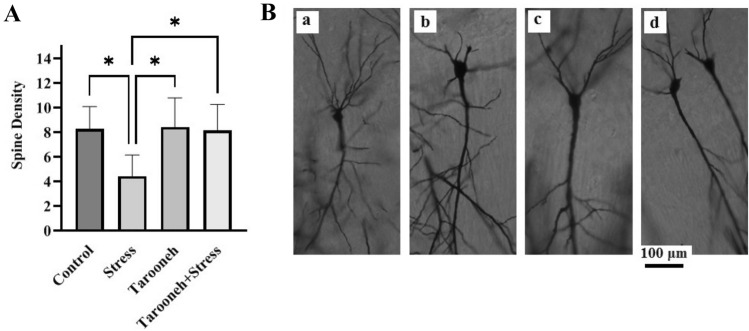
Compared to the control group (11.00 ± 0.85), 14 consecutive days of restraint stress significantly decreased the spine density (3.33 ± 0.42, F(1.96,9.83) = 16.38, P = 0.0008) in the CA1 neurons of the hippocampus. However, Tarooneh prevented this spine density loss in the stressed rats (Stress + Tarooneh) (9.33 ± 1.11) and increased the number of spines compared to the stress group (Fig. 3A). The spine density of hippocampal CA3 neurons was significantly decreased in the stress group (4.43 ± 0.65, F(1.81,10.86) = 6.45, P = 0.0158) compared to the control group (8.28 ± 0.68). Administering Tarooneh decreased the spine density loss in the pyramidal neurons in the CA3 region of the hippocampus (8.14 ± 0.8) (Fig. 4A). In the DG region of the hippocampus, the number of spines significantly decreased after 14 consecutive restraint stress (3.00 ± 0.51, F(2.45, 12.29) = 9.97, P = 0.0019) compared to the control group (6.50 ± 0.42). However, administering Tarooneh enhanced the spine density in stressed animals (Stress + Tarooneh) (5.33 ± 0.50) (Fig. 5A). In the frontal cortex, chronic restraint stress significantly reduced the number of spines (5.83 ± 0.47, F(1.83, 9.17) = 12.48, P = 0.0027) compared to the control group (11.50 ± 0.76). Administration of Tarooneh increased spine density (Stress + Tarooneh) (9.00 ± 0.77) compared to stressed animals. In unstressed animals, Tarooneh had no significant effects (11.5 ± 0.71) than the control and Stress + Tarooneh groups (Fig. 6).
Fig. 3.
A Golgi–Cox staining showed that Tarooneh extract increases neuronal spine density in the pyramidal CA1 neurons after the chronic restraint stress. B Exemplary photomicrographs of stained neurons in the hippocampal CA1 neurons. (a) Control; (b) Stress; (c) Tarooneh; (d) Stress + Tarooneh groups. Three animals in each groups were used for Golgi–Cox staining. Then the images were analyzed with the ImageJ software. The number of spine density on each neuron per 100 μm was estimated. For each group, three animals were selected, five slides were analyzed, and, of all sections, a minimum of seven neurons was selected on each rat. The scale bar represents 100 μm. All data are presented as mean ± SEM. **P = 0.0008, *as a P < 0.0052
Fig. 4.
A Golgi–Cox staining showed that Tarooneh extract increases neuronal spine density in the pyramidal CA3 neurons after the chronic restraint stress. B Exemplary photomicrographs of stained neurons in the hippocampal CA3 neurons. (a) Control; (b) Stress; (c) Tarooneh; (d) Stress + Tarooneh groups. The scale bar represents 100 μm. All data are presented as mean ± SEM. *P = 0.0158
Fig. 5.
A Golgi–Cox staining showed that Tarooneh extract increases neuronal spine density in the DG neurons after the chronic restraint stress. B Exemplary photomicrographs of stained neurons in the hippocampal DG neurons. (a) Control; (b) Stress; (c) Tarooneh; (d) Stress + Tarooneh groups. The scale bar represents 100 μm. All data are presented as mean ± SEM. *P < 0.05, **P = 0.0019
Fig. 6.
A Golgi–Cox staining showed that Tarooneh extract increases neuronal spine density in the frontal cortex neurons after the chronic restraint stress. B Exemplary photomicrographs of stained neurons in the frontal cortex neurons. (a) Control; (b) Stress; (c) Tarooneh; (d) Stress + Tarooneh groups. The scale bar represents 100 μm. All data are presented as mean ± SEM. *P = 0.0027
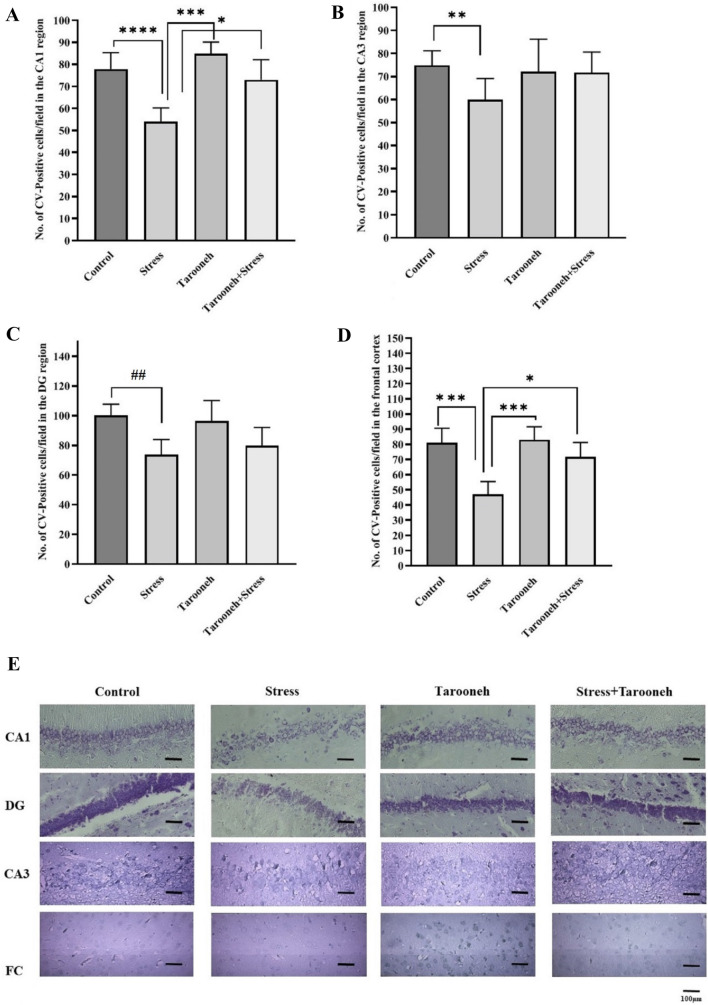
Tarooneh increases the number of CV-positive cells in the pyramidal layer of CA1 and frontal cortex in the stressed animals
To evaluate the possibility of cellular damage in the hippocampus after chronic restraint stress, histological analysis of cresyl-violet (CV) staining was performed. Evaluation of CV-positive cells showed that the 14 consecutive restraint stress significantly reduced the number of neurons in the pyramidal layer of the CA1 region (54.00 ± 2.35, F(1.70, 10.23) = 21.19, P = 0.0003) compared to the control group (77.86 ± 2.82). However, administration of Tarooneh for 14 consecutive days increased the number of CV-positive cells (73.00 ± 3.43, P < 0.05) compared to the stress group (Fig. 7A). The results showed that chronic stress significantly reduced the number of CV-positive cells in the CA3 (59.86 ± 3.50, F(1.53,9.30) = 2.71, P = 0.0065) (Fig. 7B), and DG region (73.86 ± 3.79, F(1.99,11.98) = 7.82, P = 0.0067) (Fig. 7C) compared to the control group (74.86 ± 2.37 and 100.10 ± 2.85, respectively). Results revealed that the 14 consecutive restraint stress significantly reduced the number of neurons in the frontal cortex (47.00 ± 3.21, F(2.35, 14.11) = 25.64, P < 0.0001) compared to the control group. However, administration of Tarooneh for 14 consecutive days increased the number of CV-positive cells in the frontal cortex compared to the stress group (Fig. 7D).
Fig. 7.
Quantitative analysis of neuronal damage in the different groups in the A CA1, B CA3, C DG, and D frontal cortex regions. All data are presented as mean ± SEM. *P < 0.05, **P = 0.0065, *** as P < 0.0001, **** as P = 0.0003, ##P = 0.0067. E CV staining of the CA1, CA3, DG and FC (frontal cortex) regions in the different groups. Three animals in each groups were used for CV staining. Three animals in each groups were used for CV staining. We counted ten microscopic fields at least in eight slides of each group, and the number of CV-positive cells was reported as mean ± SEM. The scale bars indicate 100 μm
Discussion
Chronic restraint stress is a common model of chronic psychoemotional stress and is used widely in neurobiological research (Jangra et al. 2020). The brain is the key organ of stress that determines threatening and stressful factors and designs physiological and behavioral responses. Some areas of the brain, including the hippocampus, amygdala, and prefrontal cortex, respond to acute and chronic stress by performing structural reconstruction, which changes behavioral and physiological reactions (McEwen 2006). The study investigated the effects of chronic restraint stress on anxiety-like behavior, cognitive impairment, spine density, and cell damage in rats, and the potential protective effects of Tarooneh.
The results of the EPM test revealed that chronic restraint stress significantly altered animals' anxiety-like behaviors (the time spent in the open arm and number of entries to the open arms decreased, and the head dipping behavior was increased). Indeed, the present results indicated anxiety-like behaviors after chronic restraint stress. In line with our results, several studies report the relation between chronic restraint stress and anxiety (Chiba et al. 2012; Moreno-Martínez et al. 2022). After that, the effects of Tarooneh on anxiety-like behavior in chronic stress were investigated. The present results demonstrated that the administration of Tarooneh significantly altered the anxiety-like behavior in stressed animals. The administration of Tarooneh led to a reduction in anxiety-like behavior, as evidenced by the results of the EPM test. Specifically, rats spent more time in the open arms and entered the open arms more frequently, while the frequency of head dipping decreased. Hence, treatment with Tarooneh produces an anti-anxiety effect induced by chronic restraint stress. To our knowledge, there is no study on the anxiolytic effects of Tarooneh in chronic restraint stress.
Different studies indicate that chronic restraint stress is involved in cognitive dysfunction (Huang et al. 2015; Zhang et al. 2021). The results of the Barnes maze showed chronic restraint stress-induced spatial memory impairments (restraint stress increased latency time, number of errors, and distance traveling in rats in the Barnes maze test) compared to the control group, and treatment with Tarooneh significantly prevented this impairment. To our knowledge, there was no study on the effect of Tarooneh in improving learning and memory in rodents.
The two main components of memory formation are the hippocampus and the frontal cortex, which are connected directly and indirectly (Place et al. 2016). Our results demonstrated that chronic restraint stress causes damage to neural spine density and synaptic plasticity in the CA1, CA3, and DG regions of the hippocampus and frontal cortex neurons. Also, the results of this study revealed that chronic restraint stress caused neural death in CA1, CA3, and DG of the hippocampus and frontal cortex. Several studies indicated that stress-induced impairment of the neural activity of the hippocampus reduced synaptic plasticity and caused cognitive impairment (Lesuis et al. 2019; Linnemann and Lang 2020; Yan et al. 2021). Hippocampus has an important role in controlling the behavior and processing of emotions and cognition in response to anxiety, depression, and fear conditions (Hernandes et al. 2021; Komleva et al. 2021). Chronic stress causes synaptic loss and neural cell death followed by neuropsychiatric symptoms, such as anxiety and cognitive impairment (Scott et al. 2015; McEwen 2017). It was reported that chronic restraint stress-induced cell death and neurodegeneration in the hippocampus (Huang et al. 2015). In chronic stress, structural and functional changes of hippocampal neurons are associated with dendritic reorganization as well as changes in the density of dendritic spines (Patel et al. 2018). In this study, we used Golgi–Cox staining to establish the neuroprotective effects of Tarooneh. The results showed that chronic restraint stress significantly reduced the spine density in CA1, CA3, and DG regions of the hippocampus as well as in frontal cortex neurons. These results are in line with the other studies demonstrating synaptic loss in chronic restraint stress (Patel et al. 2018). Also, some studies indicate that chronic stress is responsible for decreased dendritic arborization, hippocampal neuron shrinkage, change in dendrite and dendritic spines shape, and damage to neural plasticity (Schnell et al. 2012). Our results showed that the administration of Tarooneh, for 14 consecutive days, significantly increased the spine density in the CA1, CA3, and DG regions of the hippocampus and frontal cortex in the rats under chronic restraint stress. These results indicated the neuroprotective effects of Tarooneh and their preventive effects on the synaptic loss of hippocampus and frontal cortex neurons induced by chronic restraint stress. Several studies support the observed neuroprotective effects of date seed extract. For example, Kalantaripour et al. revealed that treatment with date deed extracts attenuated neuronal damage induced by middle cerebral artery occlusion in male rats. They showed that treatment with date seed extract could protect cortical neurons against cerebral-induced injuries, probably due to its antioxidant properties. Also, Yusuf et al. (2017) showed that the administration of aqueous date extracts could improve the histology of lead acetate-induced toxic effects in the cerebellum of Wistar rats. To our knowledge, there are no studies evaluating the effects of Tarooneh on neural arborization and synaptic plasticity.
Finally, the study evaluated the possibility of cellular damage in the hippocampus after chronic restraint stress and found that Tarooneh increased the number of CV-positive cells in the pyramidal layer of CA1 and frontal cortex in stressed animals. These results suggest that chronic restraint stress can lead to cellular damage in the hippocampus, specifically in the pyramidal layer of the CA1 region, CA3 region, DG region, and frontal cortex. However, the administration of Tarooneh appears to have a protective effect against this cellular damage. Overall, the results suggest that Tarooneh may have potential therapeutic benefits for stress-related disorders by improving anxiety-like behavior, cognitive impairment, and spine density loss, and reducing cellular damage. However, further research is needed to confirm these findings and determine the mechanisms underlying these effects.
Conclusion
The results of the present study suggest that Tarooneh may be effective against neuronal damage induced by chronic restraint stress by ameliorating changes in neuronal plasticity at CA1, CA3, and DG hippocampal regions and frontal cortex induced by chronic restraint stress and validated its claim as a neuroprotective agent. These findings suggest that Tarooneh extract can be a drug alternative for treating many central nervous system disorders, such as cognitive impairment and anti-anxiety drugs. However, these results are preliminary to reach therapeutic application and require further studies on safety profiles, the exact mechanism of action, and the development of clinical trials.
Acknowledgements
This study was supported by Neuroscience Sciences Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran.
Author contributions
MH: conceptualization, data curation, methodology, software, writing—original draft. SR: data curation and writing—original draft. ZJ: methodology. GHM: conceptualization, data curation, methodology, project administration, writing—original draft, resources, and supervision.
Funding
None.
Data availability
Data will be made available on request.
Declarations
Disclosure of potential conflicts of interest
The authors declare that they have no competing interests.
Research involving animals
The experimental protocol was approved by the local ethics committee of Baqiyatallah University of Medical Science (ethical code: IR.BMSU.REC.1400.089), and was in accordance with the current legislation on animal experimentation (Guide for the Care and Use of Laboratory Animals, Eighth Edition 2011).
Informed consent
Not applicable.
References
- Al-Taher AY. Possible anti-diarrhoeal effect of the date palm (Phoenix dactylifera L.) spathe aqueous extract in rats. Sci J King Faisal Univ. 2008;9(1):131–138. [Google Scholar]
- Chen Y, Andres AL, Frotscher M, Baram TZ. Tuning synaptic transmission in the hippocampus by stress: the CRH system. Front Cell Neurosci. 2012;6:13. doi: 10.3389/fncel.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuro-Psychopharmacol Biol Psychiatry. 2012;39:112–119. doi: 10.1016/j.pnpbp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Dagnino-Subiabre A. Stress and Western diets increase vulnerability to neuropsychiatric disorders: a common mechanism. Nutr Neurosci. 2021;24(8):624–634. doi: 10.1080/1028415X.2019.1661651. [DOI] [PubMed] [Google Scholar]
- de Sousa Júnior GM, Vargas HDQ, Barbosa FF, Galvão-Coelho NL. Stress, memory, and implications for major depression. Behav Brain Res. 2021;412:113410. doi: 10.1016/j.bbr.2021.113410. [DOI] [PubMed] [Google Scholar]
- Escher CM, Sannemann L, Jessen F. Stress and Alzheimer’s disease. J Neural Transm. 2019;126(9):1155–1161. doi: 10.1007/s00702-019-01988-z. [DOI] [PubMed] [Google Scholar]
- Garcia-Keller C, Carter JS, Kruyer A, Kearns AM, Hopkins JL, Hodebourg R, Kalivas PW, Reichel CM. Behavioral and accumbens synaptic plasticity induced by cues associated with restraint stress. Neuropsychopharmacology. 2021;46(10):1848–1856. doi: 10.1038/s41386-021-01074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadipour M, Kaka G, Bahrami F, Meftahi GH, Pirzad Jahromi G, Mohammadi A, Sahraei H. Crocin improved amyloid beta induced long-term potentiation and memory deficits in the hippocampal CA1 neurons in freely moving rats. Synapse. 2018;72(5):e22026. doi: 10.1002/syn.22026. [DOI] [PubMed] [Google Scholar]
- Hernandes PM, Batistela MF, Vilela-Costa HH, Sant’Ana AB, Kumpel VD, Tirapelle MC, Bom AD, de Andrade TG, Zangrossi H., Jr Role of 5-HT1A receptors in the ventral hippocampus in the regulation of anxiety-and panic-related defensive behaviors in rats. Behav Brain Res. 2021;408:113296. doi: 10.1016/j.bbr.2021.113296. [DOI] [PubMed] [Google Scholar]
- Hosseini SE. Therapeutic effects of medicinal herbs on reproductive system disorders: a review. Rep Health Care. 2018;4(3):67–76. [Google Scholar]
- Huang P, Li C, Fu T, Zhao D, Yi Z, Lu Q, Guo L, Xu X. Flupirtine attenuates chronic restraint stress-induced cognitive deficits and hippocampal apoptosis in male mice. Behav Brain Res. 2015;288:1–10. doi: 10.1016/j.bbr.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Jaggi AS, Bhatia N, Kumar N, Singh N, Anand P, Dhawan R. A review on animal models for screening potential anti-stress agents. Neurol Sci. 2011;32(6):993–1005. doi: 10.1007/s10072-011-0770-6. [DOI] [PubMed] [Google Scholar]
- Jangra A, Rajput P, Dwivedi DK, Lahkar M. Amelioration of repeated restraint stress-induced behavioral deficits and hippocampal anomalies with taurine treatment in mice. Neurochem Res. 2020;45(4):731–740. doi: 10.1007/s11064-019-02945-8. [DOI] [PubMed] [Google Scholar]
- Joëls M, Karst H, Sarabdjitsingh RA. The stressed brain of humans and rodents. Acta Physiol. 2018;223(2):e13066. doi: 10.1111/apha.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantaripour TP, Asadi-Shekaari M, Basiri M, Najar AG. Cerebroprotective effect of date seed extract (Phoenix dactylifera) on focal cerebral ischemia in male rats. J Biol Sci. 2012;12(3):180–185. doi: 10.3923/jbs.2012.180.185. [DOI] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3(6):453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kim JI, Lee JW, Lee YA, Lee DH, Han NS, Choi YK, Hwang BR, Kim HJ, Han JS. Sexual activity counteracts the suppressive effects of chronic stress on adult hippocampal neurogenesis and recognition memory. Brain Res. 2013;1538:26–40. doi: 10.1016/j.brainres.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Komleva YK, Lopatina OL, Gorina IV, Shuvaev AN, Chernykh A, Potapenko IV, Salmina AB. NLRP3 deficiency-induced hippocampal dysfunction and anxiety-like behavior in mice. Brain Res. 2021;1752:147220. doi: 10.1016/j.brainres.2020.147220. [DOI] [PubMed] [Google Scholar]
- Lee B, Shim I, Lee H, Hahm DH. Fucoidan prevents depression-like behavior in rats exposed to repeated restraint stress. J Nat Med. 2013;67(3):534–544. doi: 10.1007/s11418-012-0712-5. [DOI] [PubMed] [Google Scholar]
- Lesuis SL, Lucassen PJ, Krugers HJ. Early life stress impairs fear memory and synaptic plasticity; a potential role for GluN2B. Neuropharmacology. 2019;149:195–203. doi: 10.1016/j.neuropharm.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Li H, Park JH, Yan B, Yoo KY, Lee CH, Choi JH, Hwang IK, Won MH. Neuroprotection of Alpinia katsumadai seed extract against neuronal damage in the ischemic gerbil hippocampus is linked to altered brain-derived neurotrophic factor. Lab Anim Res. 2011;27(1):67–71. doi: 10.5625/lar.2011.27.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnemann C, Lang UE. Pathways connecting late-life depression and dementia. Front Pharmacol. 2020;11:279. doi: 10.3389/fphar.2020.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio N, Shavit-Stein E, Dori A, Blatt I, Chapman J. Prolonged systemic inflammation persistently modifies synaptic plasticity in the hippocampus: modulation by the stress hormones. Front Mol Neurosci. 2013;6:46. doi: 10.3389/fnmol.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci. 2006;8(4):367–381. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Neurobiological and systemic effects of chronic stress. Chronic Stress. 2017;1:2470547017692328. doi: 10.1177/2470547017692328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mograbi KDM, Suchecki D, da Silva SG, Covolan L, Hamani C. Chronic unpredictable restraint stress increases hippocampal pro-inflammatory cytokines and decreases motivated behavior in rats. Stress. 2020;23(4):427–436. doi: 10.1080/10253890.2020.1712355. [DOI] [PubMed] [Google Scholar]
- Mokhtari M, Sharifi E, Moghadamnia D. Effect of alcoholic extract of phoenix dactylifera spathe on histological change in testis and concentrations of LH, FSH and testosterone in male rat. Iran J Med Sci. 2007;9(4):265–281. [Google Scholar]
- Moreno-Martínez S, Tendilla-Beltrán H, Sandoval V, Flores G, Terrón JA. Chronic restraint stress induces anxiety-like behavior and remodeling of dendritic spines in the central nucleus of the amygdala. Behav Brain Res. 2022;416:113523. doi: 10.1016/j.bbr.2021.113523. [DOI] [PubMed] [Google Scholar]
- Najjar S, Pearlman DM, Devinsky O, Najjar A, Zagzag D. Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: a review of clinical and experimental evidence. J Neuroinflamm. 2013;10(1):1–16. doi: 10.1186/1742-2094-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D, Anilkumar S, Chattarji S, Buwalda B. Repeated social stress leads to contrasting patterns of structural plasticity in the amygdala and hippocampus. Behav Brain Res. 2018;347:14–324. doi: 10.1016/j.bbr.2018.03.034. [DOI] [PubMed] [Google Scholar]
- Place R, Farovik A, Brockmann M, Eichenbaum H. Bidirectional prefrontal-hippocampal interactions support context-guided memory. Nat Neurosci. 2016;19(8):992–994. doi: 10.1038/nn.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Duman R. Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Mol Psychiatry. 2020;25(3):530–543. doi: 10.1038/s41380-019-0615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16(3):313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Rahimi S, Alaei H, Reisi P, Zolphaghari B, Pourshanazari AA. Surveying the effect of hydroalcoholic tarooneh (spathe of Phoenix dactylifera) extract on anesthesia and EEG barin waves. J Chem Pharm Res. 2015;7(8):1046–1051. [Google Scholar]
- Rahimi S, Alaei H, Reisi P, Zolfaghari B, Siahmard Z, Pourshanazari A. Evaluation of the effect of hydroalcoholic extract of Phoenix dactylifera on sleep and EEG. Avi J Phytomed. 2017;7:511–518. [PMC free article] [PubMed] [Google Scholar]
- Rahimi S, Alaei H, Reisi P, Zarrin B, Siahmard Z, Pourshanazari AA. Hydroalcoholic tarooneh extract (Spathe of Phoenix dactylifera) increased sedative-hypnotic effects and modulated electroencephalography brain waves in anesthetized rats. Adv Biomed Res. 2019;8:24. doi: 10.4103/abr.abr_58_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai SN, Chaturvedi VK, Singh BK, Singh MP. Trem2 deletion reduces late-stage amyloid plaque accumulation, elevates the Aβ42: Aβ40 ratio, and exacerbates axonal dystrophy and dendritic spine loss in the PS2APP Alzheimer's mouse model. Front Aging Neurosci. 2020;12:219. doi: 10.3389/fnagi.2020.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai SN, Singh C, Singh A, Singh MP, Singh BK. Mitochondrial dysfunction: a potential therapeutic target to treat Alzheimer’s disease. Mol Neurobiol. 2020;57:3075–3088. doi: 10.1007/s12035-020-01945-y. [DOI] [PubMed] [Google Scholar]
- Roohbakhsh A, Moghaddam AH, Massoudi R, Zarrindast MR. Role of dorsal hippocampal cannabinoid receptors and nitric oxide in anxiety like behaviors in rats using the elevated plus-maze test. Clin Exp Pharmacol Physiol. 2007;34(3):223–229. doi: 10.1111/j.1440-1681.2007.04576.x. [DOI] [PubMed] [Google Scholar]
- Saeedi M, Rashidy-Pour A. Association between chronic stress and Alzheimer’s disease: therapeutic effects of Saffron. Biomed Pharmacother. 2021;133:110995. doi: 10.1016/j.biopha.2020.110995. [DOI] [PubMed] [Google Scholar]
- Sántha P, Pákáski M, Fazekas ÖC, Fodor EK, Kálmán S, Kálmán J, Janka Z, Szabó G. Restraint stress in rats alters gene transcription and protein translation in the hippocampus. Neurochem Res. 2012;37(5):958–964. doi: 10.1007/s11064-011-0688-7. [DOI] [PubMed] [Google Scholar]
- Schnell C, Janc OA, Kempkes B, Callis CA, Flügge G, Hülsmann S, Müller M. Restraint stress intensifies interstitial K+ accumulation during severe hypoxia. Front Pharmacol. 2012;3:53. doi: 10.3389/fphar.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TJ, Gould E (2013) Differential effects of stress and glucocorticoids on adult neurogenesis. Neurog Neural Plast 139–164 [DOI] [PubMed]
- Scott SB, Graham-Engeland JE, Engeland CG, Smyth JM, Almeida DM, Katz MJ, Lipton RB, Mogle JA, Munoz E, Ram N, Sliwinski MJ. The effects of stress on cognitive aging, physiology and emotion (ESCAPE) project. BMC Psychiatry. 2015;15(1):1–4. doi: 10.1186/s12888-015-0497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son H, Yang JH, Kim HJ, Lee DK. A chronic immobilization stress protocol for inducing depression-like behavior in mice. JoVE. 2019;147:e59546. doi: 10.3791/59546. [DOI] [PubMed] [Google Scholar]
- Speers AB, Cabey KA, Soumyanath A, Wright KM. Effects of Withania somnifera (Ashwagandha) on stress and the stress-related neuropsychiatric disorders anxiety, depression, and insomnia. Curr Neuropharmacol. 2021;19(9):1468. doi: 10.2174/1570159X19666210712151556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P, Tripathi PN, Sharma P, Rai SN, Singh SP, Srivastava RK, Shankar S, Shrivastava SK. Design and development of some phenyl benzoxazole derivatives as a potent acetylcholinesterase inhibitor with antioxidant property to enhance learning and memory. Eur J Med Chem. 2019;163:116–135. doi: 10.1016/j.ejmech.2018.11.049. [DOI] [PubMed] [Google Scholar]
- Tripathi PN, Srivastava P, Sharma P, Tripathi MK, Seth A, Tripathi A, Rai SN, Singh SP, Shrivastava SK. Biphenyl-3-oxo-1, 2, 4-triazine linked piperazine derivatives as potential cholinesterase inhibitors with antioxidant property to improve the learning and memory. Bioorg Chem. 2019;1(85):82–96. doi: 10.1016/j.bioorg.2018.12.017. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurol. 2007;68(24):2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- Yan Y, Xu X, Chen R, Wu S, Yang Z, Wang H, Zhang T. Down-regulation of MST1 in hippocampus protects against stress-induced depression-like behaviors and synaptic plasticity impairments. Brain Behav Immu. 2021;94:196–209. doi: 10.1016/j.bbi.2021.02.007. [DOI] [PubMed] [Google Scholar]
- Yusuf AO, Buraimoh AA, Agbon AN, Raji KB, Akpulu PS. Preliminary histological studies on the effect of aqueous fruit extract of Phoenix dactylifera L. (date palm) on lead acetate-induced cerebellar damages in Wistar rats. Afr J Cell Pathol. 2017;8(1):1–8. doi: 10.5897/AJCPATH17.001. [DOI] [Google Scholar]
- Zaqout S, Kaindl AM. Golgi–Cox staining step by step. Front Neuroanat. 2016;10:38. doi: 10.3389/fnana.2016.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Jiao W, Cui H, Sun Q, Fan H. Combined exposure of alumina nanoparticles and chronic stress exacerbates hippocampal neuronal ferroptosis via activating IFN-γ/ASK1/JNK signaling pathway in rats. J Hazard Mater. 2021;411:125179. doi: 10.1016/j.jhazmat.2021.125179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.