Abstract
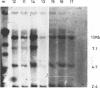
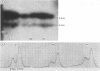
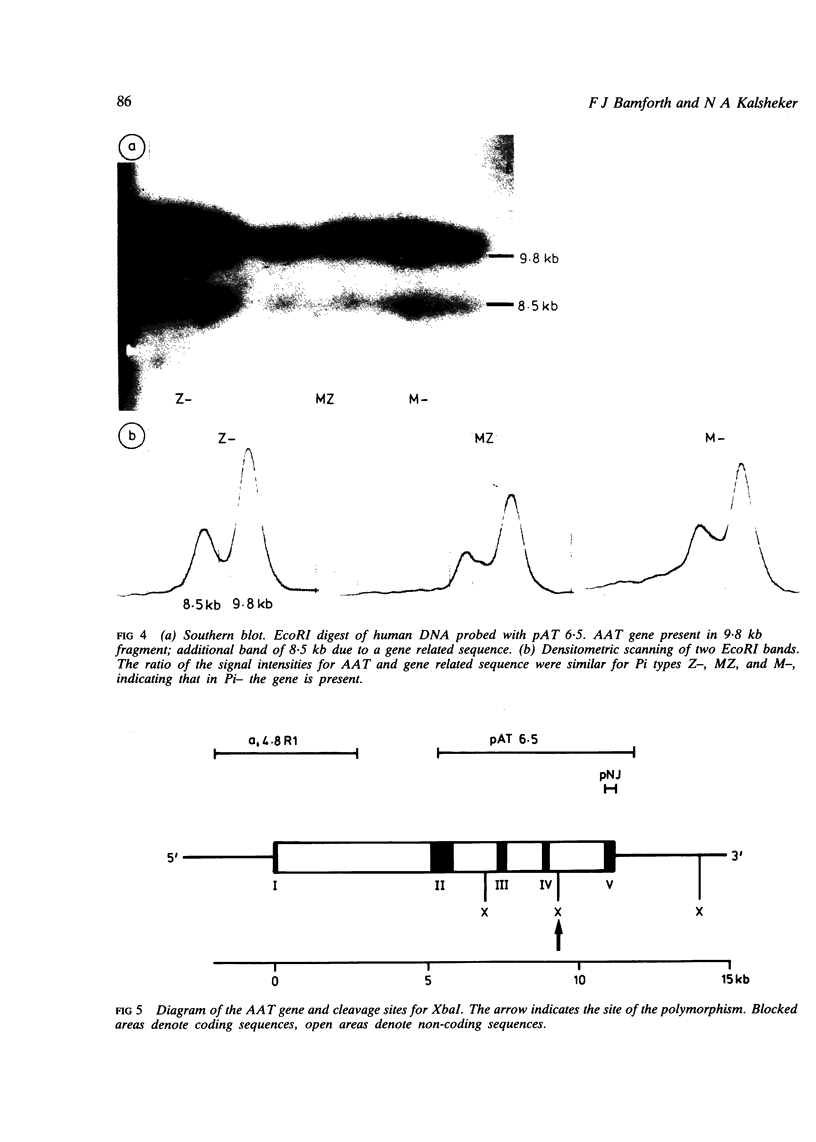
The proteinase inhibitor null (Pi-) allele is a rare cause of alpha 1 antitrypsin (AAT) deficiency. In three families, all the subjects with AAT deficiency due to PiZ- presented in early childhood with recurrent chest infections and wheezing presumably related to passive smoking. In Pi- the AAT gene is present and there is no evidence for a gene deletion. In one family a restriction fragment length polymorphism (RFLP) detected with the enzyme XbaI segregates with the Pi- allele. In a family where a consanguineous marriage occurred, the XbaI polymorphism segregates with the normal M1 allele rather than Pi-, suggesting that Pi- may have originated from M1. In contrast, a third family and 20 normal unrelated subjects do not show the RFLP.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernheim J. L., Arnaud P., Cellier C., Pokroy N., Traeger J. Apparent total alpha1-antitrypsin deficiency: report of a case. Isr J Med Sci. 1976 Jul;12(7):678–685. [PubMed] [Google Scholar]
- Burn J., Dunger D., Lake B. Liver damage in a neonate with alpha-1-antitrypsin deficiency due to phenotype PiZ null (Z-). Arch Dis Child. 1982 Apr;57(4):311–313. doi: 10.1136/adc.57.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell R. W., Jeppsson J. O., Laurell C. B., Brennan S. O., Owen M. C., Vaughan L., Boswell D. R. Structure and variation of human alpha 1-antitrypsin. Nature. 1982 Jul 22;298(5872):329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- Cook P. J. The genetics of alpha1-antitrypsin: a family study in England and Scotland. Ann Hum Genet. 1975 Jan;38(3):275–287. doi: 10.1111/j.1469-1809.1975.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Ferris B. G., Jr, Ware J. H., Berkey C. S., Dockery D. W., Spiro A., 3rd, Speizer F. E. Effects of passive smoking on health of children. Environ Health Perspect. 1985 Oct;62:289–295. doi: 10.1289/ehp.8562289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver R. I., Jr, Mornex J. F., Nukiwa T., Brantly M., Courtney M., LeCocq J. P., Crystal R. G. Alpha 1-antitrypsin deficiency and emphysema caused by homozygous inheritance of non-expressing alpha 1-antitrypsin genes. N Engl J Med. 1986 Mar 20;314(12):762–766. doi: 10.1056/NEJM198603203141207. [DOI] [PubMed] [Google Scholar]
- Hodgson I., Kalsheker N. DNA polymorphisms of the human alpha 1 antitrypsin gene in normal subjects and in patients with pulmonary emphysema. J Med Genet. 1987 Jan;24(1):47–51. doi: 10.1136/jmg.24.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsheker N. A., Warner J. T., Watkins G. L. Phenotyping alpha-1-antitrypsin (alpha 1-AT) variants by isoelectric focusing in agarose and immunoblotting. Clin Chim Acta. 1985 May 30;148(2):157–160. doi: 10.1016/0009-8981(85)90226-8. [DOI] [PubMed] [Google Scholar]
- Kidd V. J., Wallace R. B., Itakura K., Woo S. L. alpha 1-antitrypsin deficiency detection by direct analysis of the mutation in the gene. Nature. 1983 Jul 21;304(5923):230–234. doi: 10.1038/304230a0. [DOI] [PubMed] [Google Scholar]
- Klumpp T., Bieth J. G. Automated measurement of the elastase-inhibitory capacity of plasma with a centrifugal analyzer. Clin Chem. 1979 Jun;25(6):969–972. [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long G. L., Chandra T., Woo S. L., Davie E. W., Kurachi K. Complete sequence of the cDNA for human alpha 1-antitrypsin and the gene for the S variant. Biochemistry. 1984 Oct 9;23(21):4828–4837. doi: 10.1021/bi00316a003. [DOI] [PubMed] [Google Scholar]
- Rogers J., Kalsheker N., Wallis S., Speer A., Coutelle C. H., Woods D., Humphries S. E. The isolation of a clone for human alpha 1-antitrypsin and the detection of alpha 1-antitrypsin in mRNA from liver and leukocytes. Biochem Biophys Res Commun. 1983 Oct 31;116(2):375–382. doi: 10.1016/0006-291x(83)90532-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sveger T. Prospective study of children with alpha 1-antitrypsin deficiency: eight-year-old follow-up. J Pediatr. 1984 Jan;104(1):91–94. doi: 10.1016/s0022-3476(84)80599-5. [DOI] [PubMed] [Google Scholar]
- Sveger T., Thelin T. Four-year-old children with alpha 1-antitrypsin deficiency. Clinical follow-up and parental attitudes towards neonatal screening. Acta Paediatr Scand. 1981 Mar;70(2):171–177. doi: 10.1111/j.1651-2227.1981.tb05537.x. [DOI] [PubMed] [Google Scholar]