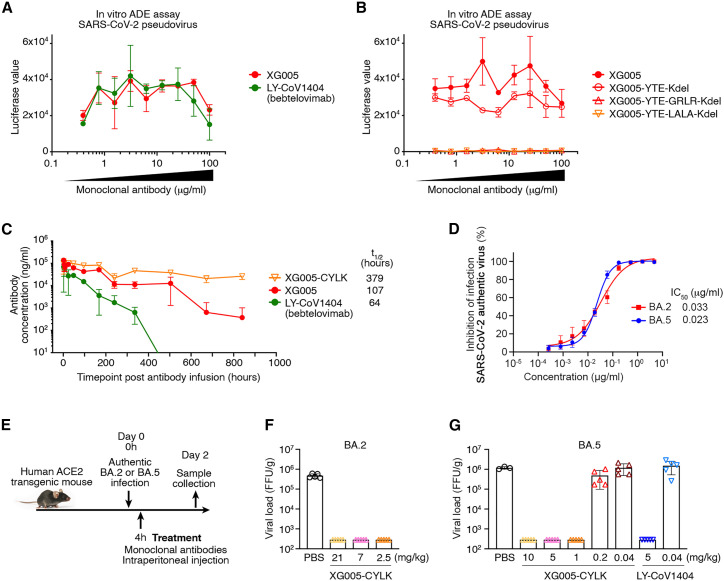
Figure 7.
XG005-CYLK is therapeutic against BA.2 and BA.5 in vivo
(A) In vitro ADE effects induced by both XG005 and its counterpart LY-CoV1404 (bebtelovimab). In vitro ADE assays were performed in the Raji cells by using luciferase-expressing SARS-CoV-2 pseudovirus. The presence of various dilutions of antibodies induced distinct levels of luciferase signal, while the luciferase signal without adding any antibody was almost zero.
(B) No ADE effect induced by the GRLR and LALA versions, but not the YTE version, of the Fc-engineered XG005 antibodies.
(C) Pharmacokinetics of single-dose mAbs, XG005, XG005-C109S-YTE-LALA-Kdel (XG005-CYLK), and LY-CoV1404 in transgenic mice, C57BL/6JSmoc, which expressed human neonatal Fc receptor (hFcRn).
(D) XG005 potently neutralizes authentic SARS-CoV-2 BA.2 and BA.5 viruses. The in vitro neutralization assays were repeated at least twice.
(E) Diagram of antibody treatment protocols for human ACE2 transgenic mice intranasally challenged with BA.2 or BA.5 viruses.
(F–G) Virus titers in lung tissues of mice collected 2 days after BA.2 (F) or BA.5 (G) viral infection. Data are presented as mean ± SD. Each group contains three to five individual mice.
See also Figures S7 and S8.