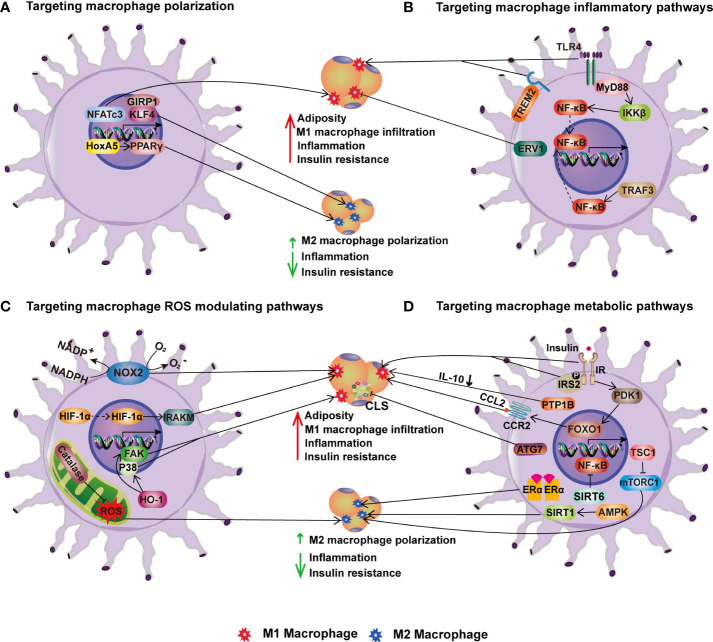
Figure 3.
Targeting macrophages for improving metabolic health. (A) Targeting macrophage polarization. In HFD-induced obesity, transcription factors NFATc3, KLF4 and its coactivator GIRP1 enhance M1 macrophage polarization and infiltration into adipose tissue, inflammation, and insulin resistance. HoxA5 and PPAR, on the other hand, increase M2 macrophage polarization and thereby ameliorate obesity-induced inflammation and insulin resistance. (B) Targeting macrophage inflammatory pathways. TLR4-MyD88-IKK signaling and TRAF3 activation enhance adipose tissue M1 macrophage infiltration, inflammation, and IR in obesity via NF-B. On contrary, overexpression of ERV1 in macrophages reduces adiposity, hepatic and adipose inflammation, and hyperglycemia caused by HFD. (C) Targeting macrophage ROS modulating pathways. NOX2, HIF-1α, and HO-1 in macrophages increase obesity-induced adiposity, inflammation, and insulin resistance, whereas catalase inhibits inflammation via increasing M2 macrophage polarization. (D) Targeting macrophage metabolic pathways. The stimulation of macrophage insulin pathways such as IR-IRS2 and PDK1-FoxO1 signaling promotes HFD-induced obesity and insulin resistance. PTP1B, an insulin signaling negative regulator, induces IR by lowering IL-10. In contrast, mTORC1 activation improves M2 macrophage polarization and protects mice from HFD-induced obesity, inflammation, and insulin resistance. In addition, the ATG7-mediated autophagy pathway reduces CLS numbers and adipose tissue inflammation in obesity. Furthermore, other metabolic pathways regulated by ERα, SIRT1 and SIRT6 enhance M2 macrophage polarization, reducing inflammation and IR in obesity.