FIGURE 5.
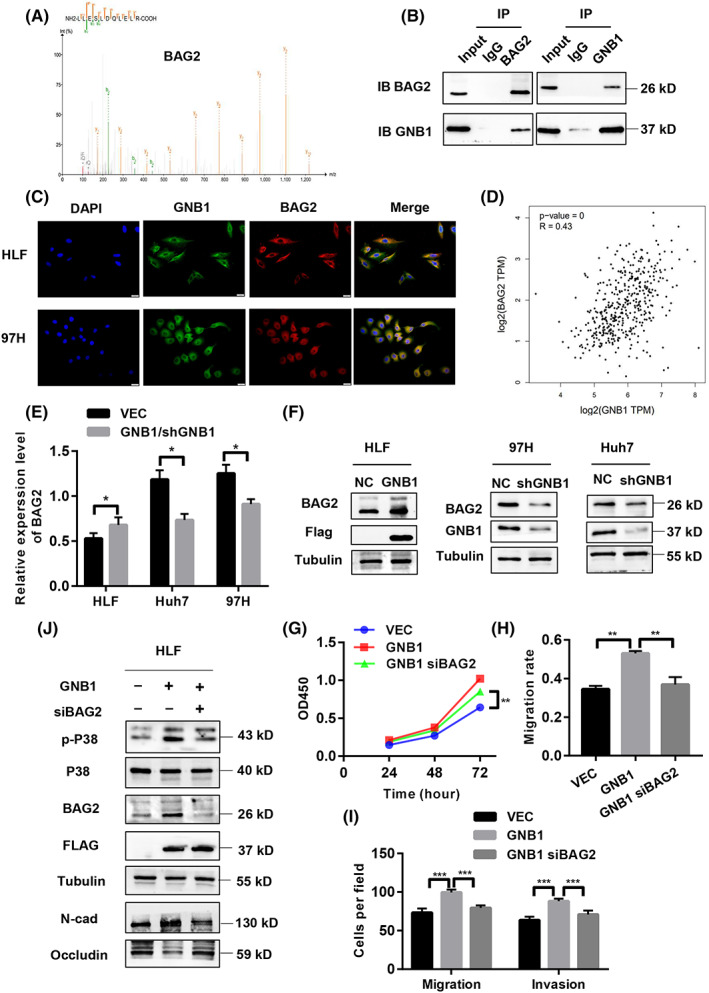
GNB1 regulate the phosphorylation of P38 through BAG2. (A) Co‐immunoprecipitation (Co‐IP) followed by liquid chromatography‐mass spectrometry (LC–MS) identified BAG2 to be a GNB1‐binding protein. (B) Co‐IP followed by western blot analyses confirmed the binding between GNB1 and BAG2 in 97H cells. (C) GNB1 and BAG2 are mainly co‐localized in cytoplasm, as demonstrated by confocal immunofluorescence analysis in HLF and 97H cells. (D) The correlation analysis in GEPIA2 shows that GNB1 and BAG2 expression levels are positively correlated. (E) The mRNA level of BAG2 was upregulated in HLF cells stably overexpressing GNB1, while 97H and Huh7cells with GNB1 knockdown showed lower BAG2 mRNA levels. (F) The protein level of BAG2 was upregulated in HLF cells stably overexpressing GNB1, while 97H and Huh7cells with GNB1 knockdown showed lower BAG2 expression levels. (G) HLF cells stably overexpressing GNB1 were transiently transfected siRNA targeting BAG2. Cell viability of indicated cells were measured by CCK‐8 assay. BAG2 knockdown reversed the increased proliferation induced by GNB1 overexpression in HLF cells. **p < 0.01, Student t test. (H, I) Wound‐healing and transwell assays were performed to assess cell migrative and invasion abilities. SiRNA targeting BAG2 treatment significantly attenuated the enhancement in cell migration and invasion induced by GNB1 overexpression. **p < 0.01, ***p < 0.001, Student t test. (J) Expression of p‐P38, P38 as well as EMT markers, N‐cadherin, and Occludin in indicated cells were examined by western blotting.
