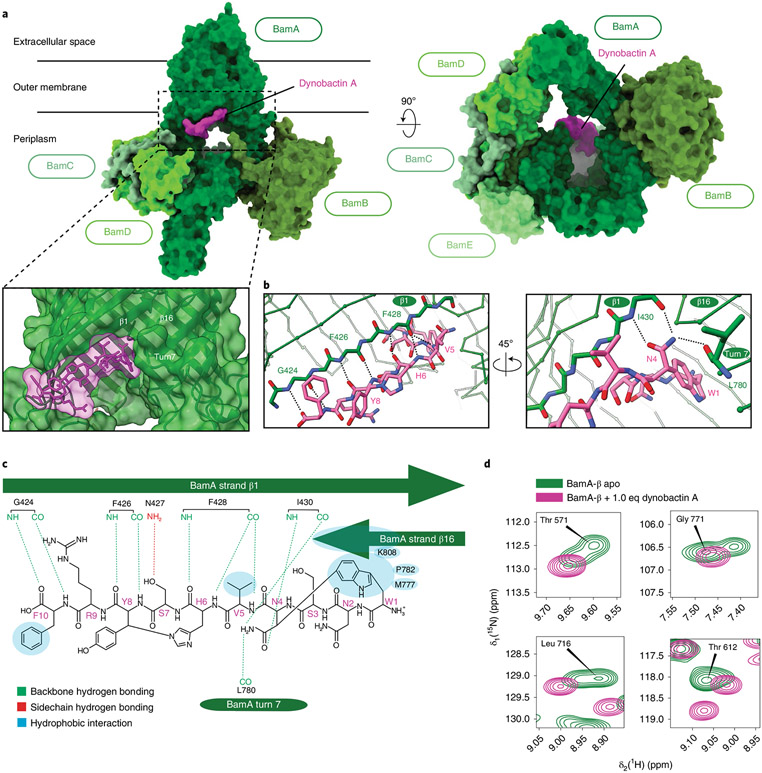
Fig. 4 ∣. Dynobactin A binds BamA lateral gate.
a, Three-dimensional structure of the BAM complex in DDM detergent micelles with bound dynobactin A, resolved by cryo-EM to a resolution of 3.6 Å. The proteins are shown as surfaces with colours as annotated. b, Close-up view of the dynobactin A binding site in a 2.5 Å crystal structure of dynobactin A bound to BamA-β. Amino acids involved in dynobactin A binding are shown in stick representation and polar contacts are shown as dashed lines: blue, nitrogen; red, oxygen; green and magenta (BamA and dynobactin, respectively), carbon. c, Schematic representation of the interactions between dynobactin A and BamA. d, Selected regions from a 2D [15N,1H]-TROSY spectrum of BamA-β in LDAO detergent micelles in the absence (green) and presence (magenta) of dynobactin A. The four selected amino acids show doublet peaks resolved into a singlet upon dynobactin A addition. Full spectra are shown in Extended Data Fig. 8.