Abstract
Objectives
To assess whether remote physical exercise interventions helped maintain function in daily life, level of physical activities, basic mobility and frailty status in pre-disabled seniors during the first Covid-19 lockdown.
Design
This is an interventional study conducted from May 2020 to May 2021.
Setting
Community-dwelling older adults in 2 Canadian cities.
Participants
84 pre-disabled seniors.
Intervention
12-week physical exercise programs (1 hour/ 3 times/ week) in kinesiologist-guided groups using Zoom or phone-supervised individual booklet-based home-program (n=44) vs. Control (usual life habits; n=40).
Measurements
Functional status in daily activities (OARS scale); Daily level of aerobic (TAPA-1) and strengthening/flexibility (TAPA-2) physical activities; Basic mobility abilities (SPPB: balance, lower limbs strength, walking speed; Timed Up-and-Go) and Frailty (SOF index) were assessed at baseline and at 3, 6, 9 and 12-month follow-ups.
Results
The participants’ mean age was 78.5 ± 7.2 and 76.5 % were women. There was a group * time effect for the OARS scale (p=0.02), the TAPA-1 (p=0.06) and the TAPA-2 (p=0.007) scores. For these outcomes, scores significantly improved during the first 3 months of follow-up and then stabilised in the intervention group whereas they remained constant in the control group over time. There was an overall time effect for the SPPB (p=0.004), the 4-m walking speed (p=0.02) and for the SOF index (p=0.004), with no between-group differences. Finally, no effect was observed for the TUG.
Conclusion
Remote home-based physical exercise interventions and monitoring during the first Covid-19 lockdown seemed to have helped maintain seniors’ level of physical activities without impacting on basic mobility abilities. Further studies are needed to identify parameters of remote exercise programs that can improve daily function and mobility in this population.
Electronic Supplementary Material
Supplementary material is available in the online version of this article at 10.1007/s12603-023-1914-1.
Key words: Exercise, gerontechnology, mobility, functional status, aging, Covid-19
Background
Physical inactivity and a sedentary lifestyle, which affect over 50% of the older people (1), lead to reductions in muscle strength and power as well as losses in mobility and functional capacities (2), creating a vicious cycle of deconditioning harmful to older adults. The Covid-19 lockdowns limited opportunities to practice physical activity (PA) (3), exacerbating inactivity and sedentary behavior (4). For instance, sedentary time increased from 5 to 8 hours per day during the first Covid-19 wave in France (5).
Counteracting physical inactivity and staying physically fit was thus critical for the elderly. Indeed, systematic reviews have shown the positive physiological effects of physical activity (PA) on the musculoskeletal system (6). Whether practiced in community groups or at home, PA improves seniors’ physical function (7, 8). A recent meta-analysis highlighted that home-based exercises improve components of physical fitness such as strength, endurance, muscle power and body balance (9). However, traditional groups and 1-on-1 in-person PA interventions with complementary exercise booklets (10) present several limitations, such as lack of access or low adherence to intervention among older adults (11). In order to overcome these shortcomings, PA using « exergames » technologies have been developed and shown effective in limiting functional losses and in improving the quality of life of the elderly (12). Although feasible, acceptable, and effective among community-dwelling older adults, such technologies involve complex electronic installations requiring technical support and high costs (13), which limit their widespread use. While counteracting physical inactivity through alternative home-based modalities of exercising was recognized as a critical need for seniors very early in the Covid-19 pandemic (9, 14), the latter also exacerbated the challenges of accessing complex technologies. As more than 70% of older adults use the Internet and have a tablet, a computer, or a smartphone (15), we relied on these current technologies to provide remote PE programs to older adults in lockdown while avoiding physical contact and risk of infection during the Covid-19 pandemic. In that context, we first showed that implementing remote exercise programs through simple web technologies or using paper-based exercise booklets with telephone supervision was feasible and acceptable for pre-disabled older adults (16). The present study aimed to assess if such programs helped maintain activity, mobility, and function in pre-disabled seniors compared to usual life habits during the first Covid-19 lockdown and in the following months.
Methods
Study design and participants
This 12-week interventional study started in May 2020 among 84 pre-disabled seniors who were previous participants of the Canadian “Cedecoms” trial (ClinicalTrial.gov: NCT03991598) (2017–2020) that tested the benefits of face-to-face exercise programs after consulting the Emergency Department (ED) for a minor injury. Participants from the last six months of the trial (October 2019–March 2020) were contacted by phone during the first Covid-19 lockdown in May 2020 to join the current study. They had all completed a post-injury exercise program by January 2020.
As described elsewhere (16), the trial participants were: 65 years and older, assessed at EDs for minor injuries not requiring hospital admission, independent in daily living activities (ADL) (eating, toileting and transfers, dressing, showering, walking, continence) in the four weeks pre-injury, and discharged home after their ED assessment. Individuals who were hospitalized or living in a long-term care facility were excluded. For the current study, those without Internet access, unable to consent, not speaking English or French, with hearing problems, or for whom the intervention was unsafe were excluded.
Participants were evaluated at baseline (T0) and at 3-(T3), 6-(T6), 9-(T9) and 12-(T12) months.
The Research Ethics Board of the “CHU de Québec-Université Laval” approved this research, and all participants gave informed consent.
Group allocation and blinding
Given the strict restrictions imposed by the Covid-19 lockdown that prevented delivering well-design RCTs, this trial used a pragmatic approach. To ensure the target number of participants, especially in the intervention, we used sequential recruitment by completing the intervention group first and then the control group in a second phase. The choice of intervention modalities (zoom groups or booklets with phone supervision) was based on participants’ preferences and availability of web tools, feasibility, and safety in their specific home environment. Due to practical considerations, the participants and the six staff kinesiologists (trainers/evaluators) were not blinded to the allocation.
Intervention
Participants in the intervention group received a 12-week PE remote program (1 hour/3-times/week) via Zoom supervised groups or weekly phone-supervised individual booklet-based home program. The study PE experts committee designed the physical exercise programs. These experts also helped develop the previous Cedecoms PE booklets used with participants doing individual programs. All PE programs were adapted to participants’ mobility and functional status and were supervised by kinesiologists. Both group and individual programs included three 4-week blocks with progressive intensity. Each session started with a 5-minute warm-up (2 repetitions of 8 stretching exercises), followed by 30 minutes of standing balance and weight-bearing strengthening (3 repetitions of 12 exercises), 10 minutes of light aerobic exercises (3 repetitions of 4 exercises), and ended with a 5-minute cooldown including stretching (1–2 repetitions of 7 exercises). Pictures of a Zoom group and individual exercise sessions are provided in Supplemental material, along with selected examples of some exercises from the first 4-week block.
Control
Participants in the control group were asked to maintain their usual lifestyle habits.
Measurements
Participants were evaluated and interviewed at baseline and every three months for one year through Zoom meetings or by phone. Our staff kinesiologists conducted all assessments for which they previously trained with the team who developed the new remote physical assessment methods (17). Assessments were not blinded.
Functional status in daily activities (ADLs)
The 14-question Older Americans Resources and Services functional scale (OARS) was used to assess independence in seven basic and seven instrumental activities of daily living (ADL/IADL). Each item is scored from 0 to 2. Total scores range from 0 to 28, with the highest scores indicating complete independence and the lowest indicating the inability to perform daily activities without help (18).
Level of physical activity (PA)
The validated 9-item Telephone Assessment of Physical Activity (TAPA) questionnaire was used to assess the daily (or most days) level of PA (19). The level of aerobic activities (TAPA-1) was assessed by asking participants to choose from a list of 7 “yes”/“no” items describing physical activities (light, moderate, vigorous) the one that best fits their situation. The total TAPA-1 scores range from 1/7 (sedentary) to 7/7 (≥ 20 minutes/day of vigorous activities, ≥ 3 days a week). Any score< 6/7 is considered suboptimal. Two additional questions assess strengthening and flexibility physical activities (TAPA-2). The TAPA-2 scores are strengthening activities ≥ once a week=1; flexibility activities ≥once a week = 2, both = 3, none =0.
Basic mobility
Three validated tests were used to assess basic mobility abilities.
- The Short Physical performance battery (SPPB) was used to evaluate physical performance (20). It includes three sub-tests: body balance, 4-meter gait speed, and leg strength (5 times chair stands). Each test is rated on a 0 to 4-point scale with a 0-score given in case of inability to do the test. Summing up the three subtests, the total SPPB scores range from 0 to 12. A total SPPB <10 is the commonly accepted threshold for functional impairment (21). In the body balance sub-test, subjects are instructed to hold three stances (joined, semi-tandem, and tandem) for 10 seconds. The 4-meter gait speed is recorded using a stopwatch. For the chair stand sub-test, the time needed to rise from a straight-backed chair five consecutive times with the arms folded across the chest is recorded. As in-person visits with participants were not allowed during the Covid-19 lockdown, kinesiologists remotely assessed the SPPB items. In parallel to the current study, Peyrusqué et al. validated the remote assessment of SPPB using Zoom (17). However, the evaluations had to be standardized for all participants, whether they were followed via Zoom or telephone. Hence, with five Zoom participants with excellent home set-ups, we determined that eight steps were required to complete the 4-m walking test and 12 for the TUG. Participants were thus instructed to walk eight steps at our “GO” signal and to count their steps out loud for the 4-m test. For the TUG test, they were instructed to rise from a chair at our “GO” signal, walk eight steps, and return to the chair, saying “OK” when seated again. When participants were well positioned for the balance tests, a “GO” was given, and a timer started for 10 seconds or until participants said: “finish”. For the 5-time chair stands, participants were given a “GO” signal, asked to count out load their stands and say “finish” once finally seated.
- 4-m walking speed: this test is a standard in physical performance evaluation (22). It was performed at the usual self-pace and fast speed. The time (in seconds) needed to walk eight steps was recorded (23). A time longer than 5 seconds (< 0.8 m/sec) on the standard 4-m test indicates a risk of mobility loss.
- Timed Up-and-Go (TUG) test (in seconds): this test which consists in raising from a chair, walking 12 steps, and sitting down again (24), is performed at a fast-paced walking speed. A time > 30 seconds indicates limited mobility and increased risk of falling, whereas a duration <20 seconds indicates appropriate mobility and subject likely independent in ADLs (25).
Frailty status
The Study of Osteoporotic Frailty index (SOF) includes three criteria: (1) unintentional 5% weight loss in the last six months, (2) inability to rise from a chair five times without using the arms, (3) low energy level (subject replying “no” to the question “Do you feel full of energy?” from the Geriatric Depression Scale (26)). Participants presenting 0,1 or ≥ 2 criteria are respectively considered robust, prefrail, or prefrail (27). Increased SOF scores are associated with increased risks of adverse health outcomes (27).
Socio-demographic and other data
Age, sex, main occupation, completed years of schooling, marital status, Body Mass Index (BMI), cognitive status (Telephone Interview for Cognitive Status-TICS-m (28)), loneliness (UCLA scale (29)) and fear of falling (Short Fall Efficacy Scale-I (29)) were assessed at baseline by phone using validated questionnaires.
Statistical analysis
The participants’ baseline characteristics were described by means ± standard deviation (SD) and frequencies. T-tests for independent samples, Chi-squared, and Fisher tests were used to compare groups at baseline. In order to assess the impact of the interventions on functional status and mobility measures, general linear models (GLM) analyses for repeated measures were used to estimate time, group, and time*group effects. Pre/post-intervention within-group differences were assessed using paired t-tests. Statistical analyses were performed using SPSS 25.0 (Chicago, IL, USA) and SAS software version 9.4 (SAS Institute, Inc, Cary, NC). Statistical significance was set at p ≤ 0.05 for all tests.
Results
Participants
Among the 214 potential subjects, 164 were eligible for this study. Among them, 80 declined participation (48.8%), and 84 subjects were included (51.2%). At baseline, there were 40 participants in controls and 44 in intervention, among whom 11 were assigned to Zoom groups and 33 to the phone-supervised booklet-based program. A total of 5 participants with a booklet-based program dropped out, along with two others in the Zoom groups who wished to continue in controls, for a total of 7 dropouts in the intervention. There were also five dropouts in controls (Figure 1). The burden and technical difficulties with remote training and/or assessments were reasons for withdrawing from the study.
Figure 1.
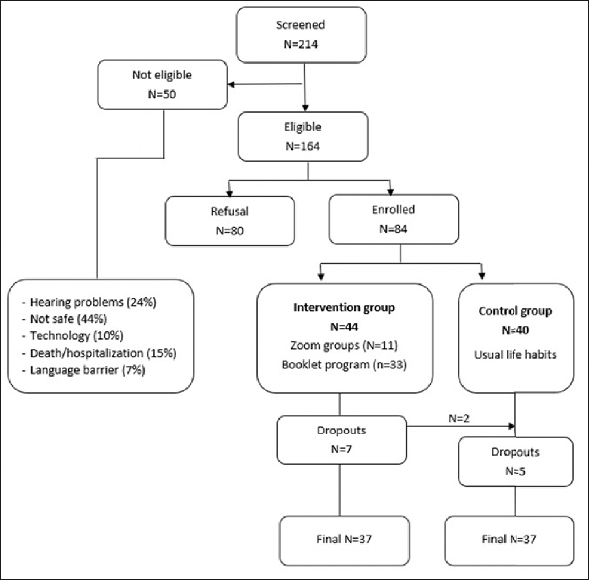
Study flow chart
Among the 44 participants in the intervention, 30 (71.4 %) were women, and their mean age was 79.2 ± 6.2 years. Among the 40 controls, 32 (82.1%) were women, and their mean age was 77.6 ± 8.1 years. At baseline, both groups were comparable regarding socio-demographic, health-related status, and physical and psychological factors (Table 1).
Table 1.
Participant’s baseline characteristics (n=84)
| Intervention group (n=44) | Control group (n=40) | p-value | |
|---|---|---|---|
| Demographic factors (n,%) | |||
| Women | 30 (71.4) | 32 (82.1) | 0.26 |
| Age (years) (mean ± SD) | 79.2 ± 6.2 | 77.6 ± 8.1 | 0.31 |
| Main occupation | |||
| Full- or part- time work | 1 (2.3) | 1 (2.5) | 0.95 |
| Retirement | 44 (100) | 39 (97.5) | 0.29 |
| Volunteer work | 3 (6.8) | 3 (7.5) | 0.90 |
| Education* | |||
| Primary school | 5 (11.4) | 4 (10.0) | 0.67 |
| High school | 8 (18.1) | 12 (30.0) | |
| College / University | 29 (66.0) | 23 (57.5) | |
| Marital status* | |||
| Married /Living with partner | 20 (45.5) | 15 (37.5) | 0.33 |
| Divorced /Widowed /Single | 23 (52.2) | 24 (60.0) | |
| Health-related status (mean ± SD) | |||
| Perceived general health | |||
| SF-12 (/100) | 68.5 ± 20.3 | 66.8 ± 19.5 | 0.71 |
| BMI (kg/m2) | 26.2 ± 4.4 | 29.3 ± 5.9 | 0.06 |
| Psychological factors (mean ± SD) | |||
| Loneliness, UCLA score (/9) | 4.2 ± 1.4 | 4.3 ± 1.6 | 0.63 |
| Cognitive status, TICS (/50) | 34.6 ± 10.7 | 36.2 ± 5.4 | 0.39 |
| Fear of falling, SFES-I (/10) | 3.4 ± 2.6 | 3.6 ± 3.5 | 0.60 |
Legend: SF-12: 12-item Short Form Survey; BMI: Body Mass Index; TICS: Telephone Interview for Cognitive Status; SFES-I: Short Falls Efficacy Scale International; *Due to missing values the total do not add to 100%
Details on the adherence rate in the intervention were provided elsewhere (16). Briefly, 10% of participants exercising in Zoom groups followed 2/12-week of their program, 10% did 5–6/12 weeks, 20% did 9–10/12 weeks, and 60% did 11–12/12 weeks. Among participants exercising with a booklet, 3.3% did 1/12-week of their program, 10% did 3–4/12 weeks, 6.7% did 7–8/12 weeks, 6.7% did 9–10/12 weeks, and 73.3% did 11–12/12 weeks.
Effects of the intervention on mobility and functional status
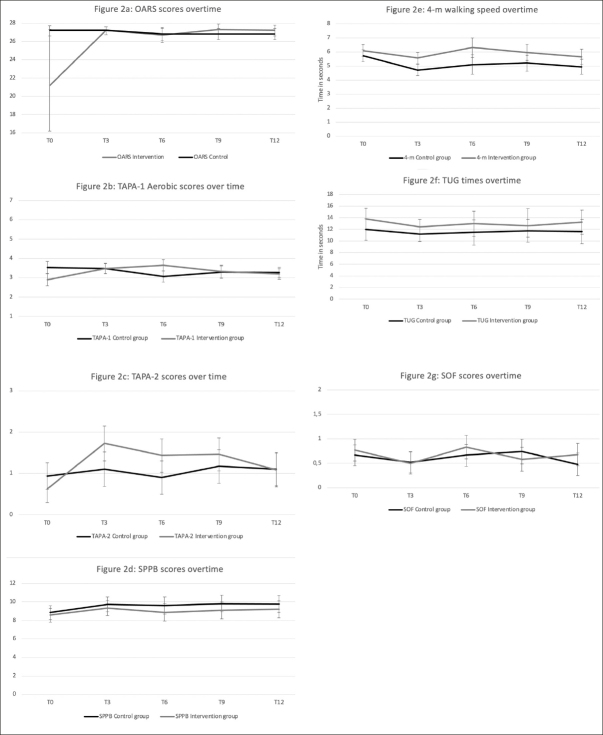
Figure 2 presents the mobility and functional outcomes at each follow-up time, according to groups. There was a significant group*time effect for the OARS scores (p=0.02). As shown in Figure 2a, the OARS score significantly improved from baseline (21.2/28) to T3 (27.2/28) in the intervention group and remained stable beyond this point. In the control group, the OARS score remained constant during the study.
Figure 2.
Evolution of the outcomes overtime, according to intervention and control groupst
OARS: Older American Resource Scale of functional status; TAPA-1: Telephone Assessment of Physical Activities-aerobic; TAPA-2: Telephone Assessment of strengthening and flexibility Physical Activities; SPPB: Short Physical Performance Battery; TUG: Timed Up-and-Go test; SOF: Study of Osteoporose Frailty index.
There was a group*time effect for the TAPA-1 aerobic scores (p = 0.002). There was no trend in the control group scores, while there was a quadratic one in the intervention group (p=0.0001). As shown in Figure 2b, the TAPA-1 scores improved over the first six months in the intervention group (from 2.9 to 3.7/7) and then decreased until the last follow-up. There was also a group*time effect (p=0.007) in this group for the TAPA-2 strengthening/flexibility scores with a quadratic trend (p<0.0001). These TAPA-2 scores increased over the first three months, plateaued for six months, and declined in the last three months remaining at a higher level than the initial measures (Figure 2c).
Regarding basic mobility measures, there was a time effect for the SPPB total scores (p=0.004) but no between-group differences. Globally, SPPB scores improved in both groups in the first three months, then slightly dropped at six months and stabilized slightly above initial measures until T12 (Figure 2d). There was also a time effect for the 4-m walking speed (p<0.0001) with some group differences (p=0.02). As shown in Figure 2e, baseline 4-m walking speed was somewhat better in the intervention group, and this gap increased over the follow-up time points. There were no time effects (p=0.07) or between-groups differences for the TUG measures (Figure 2f).
Finally, regarding the SOF frailty scores, there was an overall time effect (p=0.004) with no between-group differences (Figure 2g).
Discussion
The present study aimed to assess if remote-based intervention in physical exercises helped counteract sedentary/inactivity associated with the Covid-19 lockdown and maintaining mobility and daily functioning in pre-disabled seniors.
Globally, besides the T0-T3 improvements in OARS mean scores in the intervention group, there were no other significant changes in daily functioning activities in either group. This could be explained by the already good baseline scores in controls (26/28). This improvement of OARS means in intervention might also be due to the early dropout of participants with low initial values, which, given our small Ns, could have had a strong impact on means at the subsequent follow-ups. In a meta-analysis by Tak et al. (30) involving 8500 subjects, the relative risk of progressing to ADL disability in older adults with a medium/high PE level compared to those with low levels was 0.55 (p<001) (30). Although our intervention participants reported higher physical activity levels than controls, this did not translate into overall better ADL performances. In fact, ADL functioning had already improved to good levels with the exercise programs from the Cedecoms trial six months before lockdown, leaving almost no room for further improvements (31).
The study’s most important findings are the significant improvements in aerobic and strength/flexibility physical activity levels over time with remote exercise programs based on three sessions/week for 12 weeks. Whatever modalities (Zoom groups or booklet with phone supervision), the effect of programs on TAPA scores lasted beyond their 12-week duration. This is important since increased physical activity helps maintain health (30) and delay the decline in function in older adults (32).
Interestingly, trends in basic mobility measures (SPPB test, 4-m walking speed, TUG test) were similar in both groups. A potential learning effect could partly explain this as participants performed the same assessments every three months over a year (33) but mainly, again, because they had completed exercise programs six months before the lockdown, during which their mobility improved (31). As the Covid-19 lockdown was gradually lifted over the study period allowing seniors to move around again might also have impacted mobility measures. Recent meta-analyses showed improvements in SPPB scores (34), improvements of 0.92 seconds on the Timed Up-and-Go test (35), and functional and clinical improvements in usual and fast gait speed (36) among participants who underwent face-to-face multimodal physical exercises compared to controls. Besides earlier gains from the previous trial, the mobility measures of many participants in the current study remained below the thresholds for fully independent mobility and had room for improvement. In that sense, remotely providing effective exercise programs for pre-disabled seniors is a real challenge compared to face-to-face modalities. Indeed, as mentioned by our staff kinesiologists (16), difficulties in seeing the whole body of participants in Zoom, difficulties in assessing intensity and pain and in providing proper feedback through Zoom or by phone, and participants feeling unsafe are all reasons that can make programs sub-optimal.
Finally, the frailty scores varied significantly over the study period regardless of the groups, remaining within the robust-prefrail range. Evidence suggests that physical activity is one of the best non-pharmacological interventions to prevent and manage frailty among older adults (37). However, according to the systematic review by Silva et al. (38) the most effective exercise program still needs to be identified. Since, to date, there are very few studies on remote exercise programs in pre-disabled older adults, their effect on frailty remains to be determined. Moreover, even in face-to-face interventions, frailty is assessed by a wide array of indices, and PE parameters vary a lot, making studies difficult to compare and conclusions difficult to draw.
Our results add to a growing literature focused on investigating remote PE interventions. However, many limitations must be acknowledged. First, our inclusion criteria (volunteer subjects from the previous Cedecoms trial, access to computers, tablets, or smartphones) and exclusion criteria (hearing difficulties preventing Zoom or telephone use, safety issues) make our sample non-representative of the general older adult population at home. In fact, safety issues with remote interventions prevented participation in 44% of the potentially eligible subjects who were frailer when they could have greatly benefited from exercising. Integrating such frailer older adults in remote exercise programs remains a true challenge, even for seasoned kinesiologists like ours. On the other hand, as in-person research recruitment of older persons was forbidden during the first Covid-19 lockdown and throughout the year 2020–21 in our province, enrolled participants in the current study were already known to our team as they had completed exercise programs of the «Cedecoms» trial by January 2020 in which they had already improved their mobility. This also means they were not physically inactive compared with potential new participants seeking ED consulting after minor injuries. It also mainly explains why there were no between-group differences in our ADL and mobility measures. Again, because restrictions imposed by Covid-19 waves in 2020–21 made well-design RCTs challenging to implement, this study was non-randomized and non-blinded, which could have impacted our results. As the Covid-19 lockdown was gradually lifted over the study period, we measured neither the presence of cointerventions nor the resumption of previous activities other than through the TAPA measures; this may have also impacted results toward the no between-group differences in mobility measures.
Another limitation may be that even though the programs’ contents were the same, 11 participants exercised via Zoom groups, and 33 did individual exercises with booklets. We did examine training effects according to the type of intervention at mid-study (6 months). Given our small Ns, and as the results on mobility measures were basically the same, we thus chose to present the results on the interventions altogether vs controls. Our small sample size also limits our results’ statistical power and generalizability to larger older adult populations. Finally, measurement biases may have occurred because of the online and telephone modalities of assessments. Indeed, online assessments could affect the reliability and reproducibility of the tests (larger margin of errors, viewing angles, lack of postural control, response times of participants) in pre-disabled seniors, even if a recent publication highlighted that remotely assessing functional capacities and muscle function seems as reliable and valid as a face-to-face assessment in community-living older adults (17).
Almost all studies on the subject reported negative impacts on physical activity and well-being in people with physical impairments during the first wave of the Covid-19 pandemic (39), highlighting the importance of supporting such populations in times of crisis and isolation like Covid-19. While face-to-face physical exercises help counteract physical inactivity and maintain health and mobility in pre-disable older adults, it was not possible to implement such intervention during the first year of the pandemic. We thus relied on remote modalities to achieve this. While our study had many limitations, and although our remote interventions did not translate into improved mobility, many lessons were learned. Most importantly, from a clinical perspective, implementing remote physical exercises was only possible because our kinesiologists were well-seasoned and previously knew the participants. With this a priori knowledge, they were able to screen those for whom remote interventions were safe and adapt and personalized programs for those enrolled, taking into account their home and family environments.
Conclusion
Remote physical exercise using live Zoom groups or booklets with regular and personalized follow-ups during the Covid-19 lockdowns helped maintain physical activity levels without impacting basic mobility measures in pre-disabled older adults. Further, large-scale studies are needed to identify parameters of remote exercise programs that can improve ADL function and mobility in this population.
Electronic supplementary material
Supplementary material, approximately 4.42 MB.
Acknowledgments
Authors wish to thank Mr. Pierre-Hugues Carmichael, MSc, statistician at the Centre d’Excellence sur le Vieillissement de Québec (CEVQ).
Funding
This study was funded by the Fonds de Recherche du Québec–Santé (FRQ-S), the Quebec Network for Research on Aging (RQRV) and the Canadian Institutes for Health Research (CIHR-PJT 148877).
Ethical standards
All procedures performed in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Cunningham C, O’Sullivan, Caserotti P, Tully MA. Consequences of physical inactivity in older adults: A systematic review of reviews and meta-analyses. Scandinavian Journal of Medicine & Science in Sports. 2020;30(5):816–827. doi: 10.1111/sms.13616. [DOI] [PubMed] [Google Scholar]
- 2.Bouchard DR, Janssen I. Dynapenic-obesity and physical function in older adults. The journals of Gerontology Series A, biological sciences and medical sciences. 2010;65(1):71–7. doi: 10.1093/gerona/glp159. [DOI] [PubMed] [Google Scholar]
- 3.Meyer J, Herring M, McDowell C, et al. Joint prevalence of physical activity and sitting time during COVID-19 among US adults in April 2020. Preventive medicine reports. 2020;20:101256. doi: 10.1016/j.pmedr.2020.101256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubertin-Leheudre M, Rolland Y. The Importance of Physical Activity to Care for Frail Older Adults During the COVID-19 Pandemic. Journal of the American Medical Directors Association. 2020;21(7):973–976. doi: 10.1016/j.jamda.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambonniere C, Lambert C, Tardieu M, et al. Physical Activity and Sedentary Behavior of Elderly Populations during Confinement: Results from the FRENCH COVID-19 ONAPS Survey. Experimental Aging Research. Apr 7 2021:1–13. doi:10.1080/0361073x.2021.1908750 [DOI] [PubMed]
- 6.Dulac MC, Aubertin-Leheudre M. Exercise: An Important Key to Prevent Physical and Cognitive Frailty. The Journal of Frailty & Aging. 2016;5(1):3–5. doi: 10.14283/jfa.2015.72. [DOI] [PubMed] [Google Scholar]
- 7.Chan DC, Tsou HH, Yang RS, et al. A pilot randomized controlled trial to improve geriatric frailty. BMC geriatrics. 2012;12:58. doi: 10.1186/1471-2318-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izquierdo M, Merchant RA, Morley JE, et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. The Journal of Nutrition, Health & Aging. 2021;25(7):824–853. doi: 10.1007/s12603-021-1665-8. [DOI] [PubMed] [Google Scholar]
- 9.Chaabene H, Prieske O, Herz M, et al. Home-based exercise programmes improve physical fitness of healthy older adults: A PRISMA-compliant systematic review and meta-analysis with relevance for COVID-19. Ageing Research Reviews. 2021;67:101265. doi: 10.1016/j.arr.2021.101265. [DOI] [PubMed] [Google Scholar]
- 10.Liu-Ambrose T, Donaldson MG, Ahamed Y, et al. Otago home-based strength and balance retraining improves executive functioning in older fallers: a randomized controlled trial. Journal of the American Geriatrics Society. 2008;56(10):1821–30. doi: 10.1111/j.1532-5415.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- 11.Choi SD, Guo L, Kang D, Xiong S. Exergame technology and interactive interventions for elderly fall prevention: A systematic literature review. Applied Ergonomics. 2017;65:570–581. doi: 10.1016/j.apergo.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Buckinx F, Waters DL, Aubertin-Leheudre M. Letter to the Editor: Implementing Home-Based Exercise Technology in a Nursing Home: Does MCI Status Matter? The Journal of Frailty & Aging. 2021;10(1):77–78. doi: 10.14283/jfa.2020.34. [DOI] [PubMed] [Google Scholar]
- 13.Vaportzis E, Clausen MG, Gow AJ. Older Adults Perceptions of Technology and Barriers to Interacting with Tablet Computers: A Focus Group Study. Frontiers in Psychology. 2017;8:1687. doi: 10.3389/fpsyg.2017.01687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machado CLF, Pinto RS, Brusco CM, Cadore EL, Radaelli R. COVID-19 pandemic is an urgent time for older people to practice resistance exercise at home. Experimental Gerontology. 2020;141:111101. doi: 10.1016/j.exger.2020.111101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Boekel LC, Peek ST, Luijkx KG. Diversity in Older Adults’ Use of the Internet: Identifying Subgroups Through Latent Class Analysis. Journal of Medical Internet Research. 2017;19(5):e180. doi: 10.2196/jmir.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckinx F, Aubertin-Leheudre M, Daoust R, et al. Feasibility and Acceptability of Remote Physical Exercise Programs to Prevent Mobility Loss in Pre-Disabled Older Adults during Isolation Periods Such as the COVID-19 Pandemic. The Journal of Nutrition, Health & Aging. 2021;25(9):1106–1111. doi: 10.1007/s12603-021-1688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peyrusqué E, Granet J, Pageaux B, Buckinx F, Aubertin-Leheudre M. Assessing Physical Performance in Older Adults during Isolation or Lockdown Periods: Web-Based Video Conferencing as a Solution. The Journal of Nutrition, Health & Aging. 2022;26(1):52–56. doi: 10.1007/s12603-021-1699-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCusker J, Bellavance F, Cardin S, Belzile E. Validity of an activities of daily living questionnaire among older patients in the emergency department. Journal of Clinical Epidemiology. 1999;52(11):1023–30. doi: 10.1016/S0895-4356(99)00084-0. [DOI] [PubMed] [Google Scholar]
- 19.Mayer CJ, Steinman L, Williams B, Topolski TD, LoGerfo J. Developing a Telephone Assessment of Physical Activity (TAPA) questionnaire for older adults. Preventing Chronic Disease. 2008;5(1):A24. [PMC free article] [PubMed] [Google Scholar]
- 20.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of Gerontology. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- 21.Gawel J V, Collins J, et al. The short physical performance battery as a predictor for long term disability or institutionalization in the community dwelling population aged 65 years old or older. Physical Therapy Reviews. 2012;17:37–44. doi: 10.1179/1743288X11Y.0000000050. [DOI] [Google Scholar]
- 22.Goldberg A, Schepens S. Measurement error and minimum detectable change in 4-meter gait speed in older adults. Aging Clinical and Experimental Research. 2011;23(5–6):406–12. doi: 10.1007/BF03325236. [DOI] [PubMed] [Google Scholar]
- 23.Dulac MC, Pion CH, Lemieux F, et al. Differences in muscle adaptation to a 12-week mixed power training in elderly men, depending on usual protein intake. Experimental Gerontology. 2018;104:78–85. doi: 10.1016/j.exger.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society. 1991;39(2):142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 25.Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go” test. Archives of Physical Medicine and Rehabilitation. 1986;67(6):387–9. [PubMed] [Google Scholar]
- 26.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 27.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Archives of Internal Medicine. 2008;168(4):382–9. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 28.Knopman DS, Roberts RO, Geda YE, et al. Validation of the telephone interview for cognitive status-modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology. 2010;34(1):34–42. doi: 10.1159/000255464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kempen GI, Yardley L, van Haastregt JC, et al. The Short FES-I: a shortened version of the falls efficacy scale-international to assess fear of falling. Age and Ageing. 2008;37(1):45–50. doi: 10.1093/ageing/afm157. [DOI] [PubMed] [Google Scholar]
- 30.Tak E, Kuiper R, Chorus A, Hopman-Rock M. Prevention of onset and progression of basic ADL disability by physical activity in community dwelling older adults: a meta-analysis. Ageing Research Reviews. 2013;12(1):329–38. doi: 10.1016/j.arr.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Sirois M. L’exercice pour prévenir le déclin fonctionnel et la mobilité des personnes âgées évaluées aux services d’urgence pour des blessures traumatiques mineures. l’Année Gérontologique; 2022;36(1)
- 32.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Preventing Chronic Disease. 2006;3(4):A118. [PMC free article] [PubMed] [Google Scholar]
- 33.Freire AN, Guerra RO, Alvarado B, Guralnik JM, Zunzunegui MV. Validity and reliability of the short physical performance battery in two diverse older adult populations in Quebec and Brazil. Journal of Aging and Health. 2012;24(5):863–78. doi: 10.1177/0898264312438551. [DOI] [PubMed] [Google Scholar]
- 34.Papalia GF, Papalia R, Diaz Balzani LA, et al. The Effects of Physical Exercise on Balance and Prevention of Falls in Older People: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. Aug 11 2020;9(8)doi:10.3390/jcm9082595 [DOI] [PMC free article] [PubMed]
- 35.Chase JD, Phillips LJ, Brown M. Physical Activity Intervention Effects on Physical Function Among Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. Journal of Aging and Physical Activity. 2017;25(1):149–170. doi: 10.1123/japa.2016-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hortobágyi T, Lesinski M, Gäbler M, VanSwearingen JM, Malatesta D, Granacher U. Effects of Three Types of Exercise Interventions on Healthy Old Adults’ Gait Speed: A Systematic Review and Meta-Analysis. Sports Medicine (Auckland, NZ) 2015;45(12):1627–43. doi: 10.1007/s40279-015-0371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. Journal of the American Medical Directors Association. 2013;14(6):392–7. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva RB, Aldoradin-Cabeza H, Eslick GD, Phu S, Duque G. The Effect of Physical Exercise on Frail Older Persons: A Systematic Review. The Journal of Frailty & Aging. 2017;6(2):91–96. doi: 10.14283/jfa.2017.7. [DOI] [PubMed] [Google Scholar]
- 39.de Boer DR, Hoekstra F, Huetink KIM, Hoekstra T, Krops LA, Hettinga FJ. Physical Activity, Sedentary Behavior and Well-Being of Adults with Physical Disabilities and/or Chronic Diseases during the First Wave of the COVID-19 Pandemic: A Rapid Review. International Journal of Environmental Research and Public Health. Jun 11 2021;18(12) doi:10.3390/ijerph18126342 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material, approximately 4.42 MB.