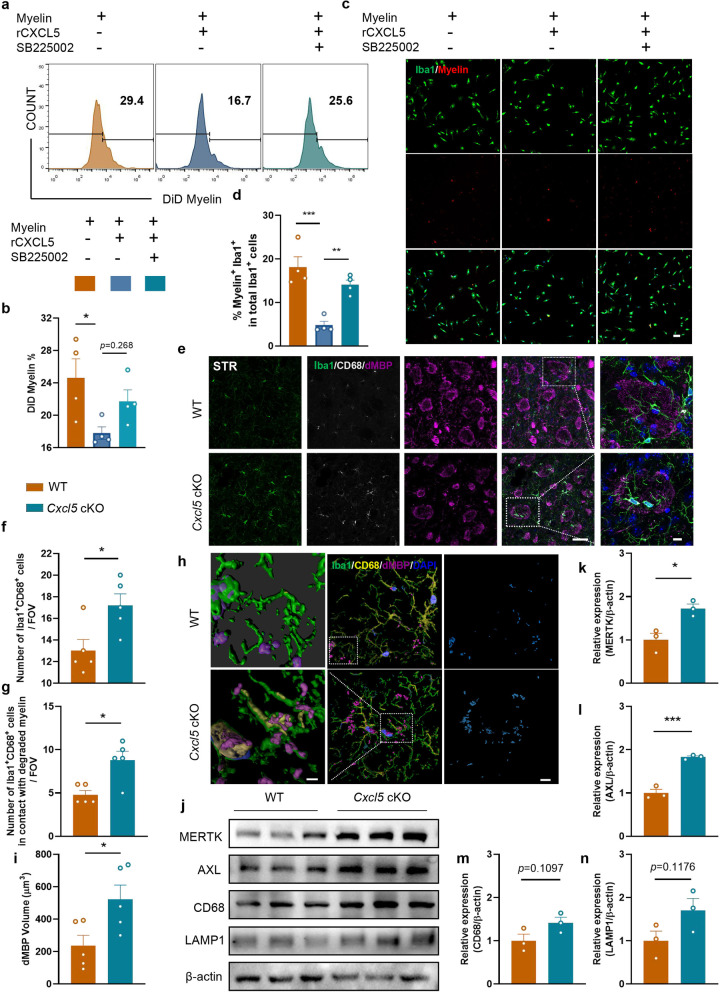
Fig. 6.
CXCL5 hindered microglia-mediated clearance of myelin debris. a, b Primary microglia were pretreated with rCXCL5 or rCXCL5 + SB225002 for 0.5 h, then incubated with myelin debris (0.01 mg/ml) stained with DiD for 1 h, primary microglia were collected (a) and the ratio of microglia phagocytosing myelin debris (DiD+ cells) among total cells were conducted by the flow cytometer (b). n = 4 per group. c, d Primary microglia were pretreated with rCXCL5 or rCXCL5 + SB225002 for 0.5 h, and then incubated with myelin debris (0.01 mg/ml) stained with DiI for 0.5 h. c Representative images for Iba-1 (green) and Myelin (red) double immunostaining. Scale bar = 50 μm. d Quantification of the ratio of Myelin+Iba-1+ cells among total Iba-1+ cells. n = 4 per group. e–i Immunostaining of Iba-1 (green)/dMBP (pink)/CD68 (grey)/DAPI (blue) in STR at 2 months after BCAS (e). Scale bar = 50 μm (left) and 20 μm (right). Quantification in the FOV in 50 μm images. f Quantification of Iba-1+CD68+ cell numbers in the WT and Cxcl5 cKO groups. g Quantification of Iba-1+CD68+ cell numbers adhered to dMBP. n = 5 mice per group. h Imaris-rendered Iba-1 (green), dMBP (pink) and CD68 (yellow) with the completely localized dMBP-CD68-Iba-1 (engulfed) channel in cyan. Scale bar = 3 μm(left) and 10 μm (middle and right). i Quantification of the average volume of dMBP in per Iba-1+CD68+ cell in the WT and Cxcl5 cKO groups. n = 5 per group. j–n Representative immunoblot bands (j) and quantification of MERTK (k), AXL (l), CD68 (m) and LAMP1 (n) normalized to β-actin in the CC. n = 3 per group. All data were presented as the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ns means no significance. One-way ANOVA with Tukey’s post-hoc for b and d. Mann–Whitney test for g. Student’s t-test for the others