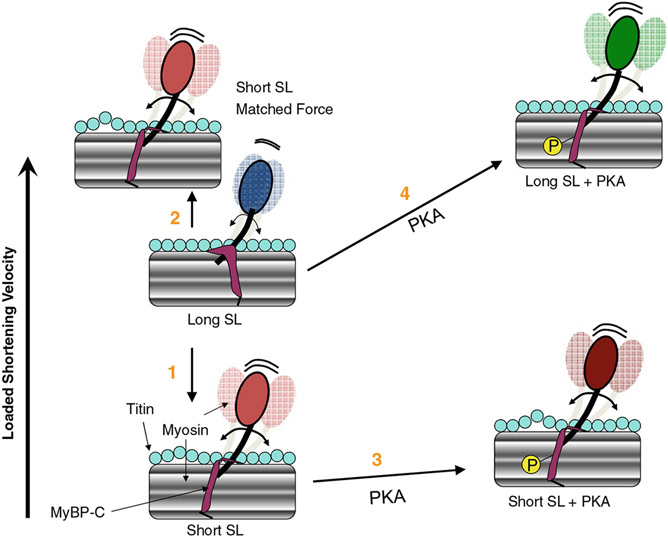
Fig. 2.
Conceptual model of interplay between sarcomere length (SL), PKA-mediated phosphorylation (P) and loaded shortening in cardiac myofilaments. (1) Loaded shortening is decreased at short SL due to decreased numbers of strongly bound cross-bridges to maintain activation of the thin filament due, in part, to increased lattice spacing. (2) If force and, presumably, thin filament activation levels are matched, loaded shortening is actually faster at short SL. Faster loaded shortening at short SL (at matched force) is illustrated to result from titin becoming less taut, which may reduce the mechanical impedance on the myosin cross-bridge (perhaps mediated by titin’s interaction with MyBP-C on the thick filament, which in turn increases myosin head radial and azimuthal mobility). This may lead to faster cross-bridge cycling by the more flexible cross-bridges being better able to maintain thin filament activation and thus allowing more cross-bridges to work against a fixed load. (3) With PKA, loaded shortening appears to be minimally affected at short SL but is faster at long SL (4), perhaps via phosphorylation of MyBP-C, which may increase the flexibility of cross-bridges resulting in more cooperative activation of the thin filament so more cross-bridges are available to work against a given load especially early in the cardiac twitch contraction. The PKA effect is greater at long SL than short SL because cross-bridge flexibility is already increased at short SL due to reduced titin strain