Figure 3. Single cell genomics reveal cell type specific perturbations in sensing and regulating neural activity in AD.
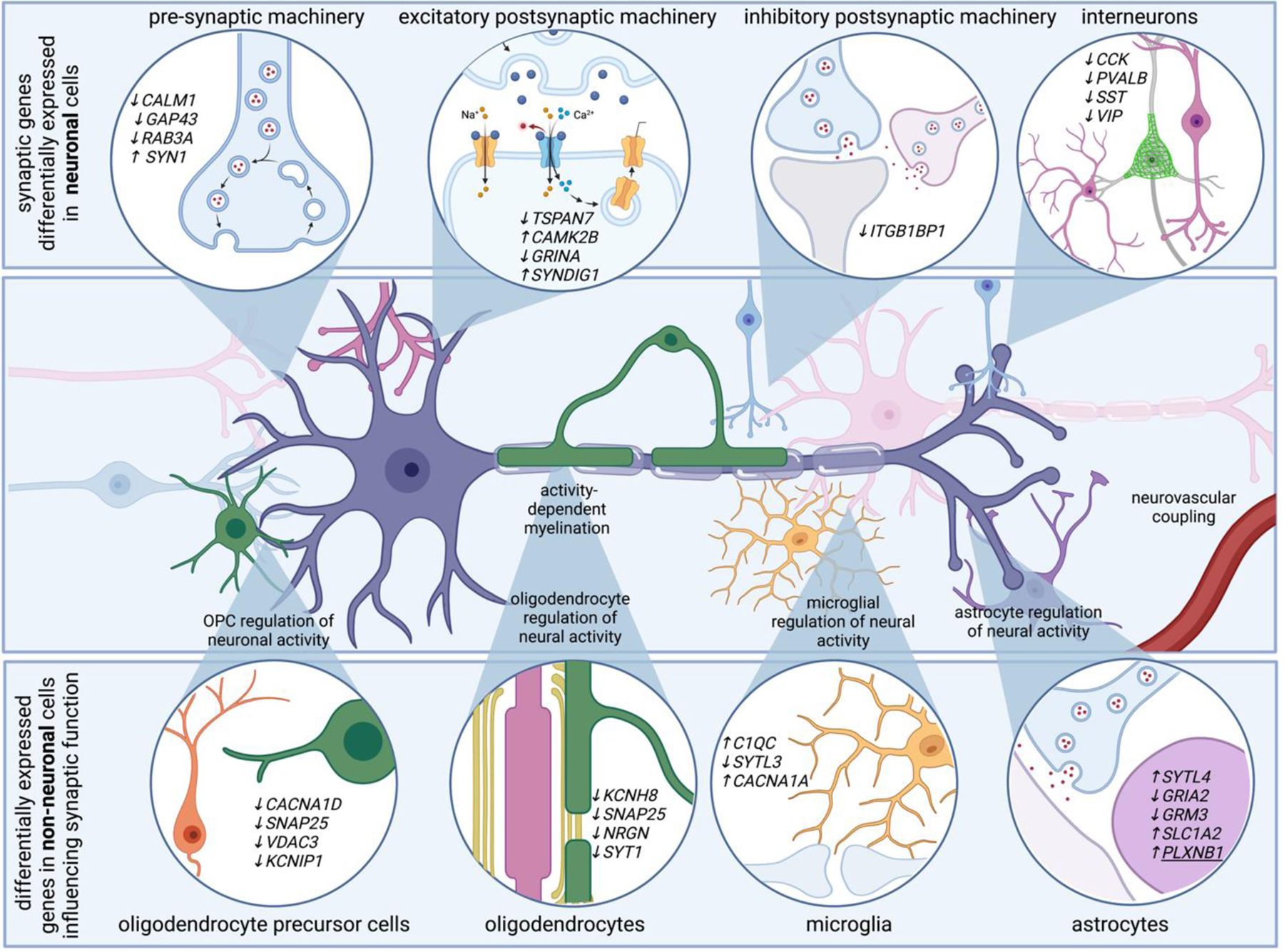
Neurons account for the vast majority of differentially expressed genes in AD. Genes related to pre-synaptic, post-synaptic, and inhibitory synaptic machinery emerge in single transcriptomes of AD neurons. For example, AD neurons upregulate SYN1, a gene that encodes synapsin 1, critical for synaptic vesicle function, and downregulate TSPAN7, which encodes a tetraspanin thought to regulate post-synaptic dendritic spine structure. Transcriptional programs associated with altered electrical properties may be associated with neuronal vulnerability to AD. Additionally, non-neuronal cells modulate genes that are involved in synaptic function. For example, genes related to synaptotagmin related genes are differentially expressed in astrocytes, oligodendrocytes, oligodendrocyte precursor cells, and microglia. Several differentially expressed genes in non-neuronal cells converge on pathways that ultimately influence neuronal function, such as genes related to synaptic pruning and activity-dependent ion channels. For example, voltage gated ion channels, which might help non-neuronal cells sense neuronal activity, are also modulated in multiple cell types. These highlight how many brain cell types are involved in sensing and regulating neural activity, and suggest neural circuit dysfunction in AD is likely the consequence of multi-cellular signaling cascades.
