Figure 5. Molecular programs adopted in AD astrocytes revealed by single cell genomics.
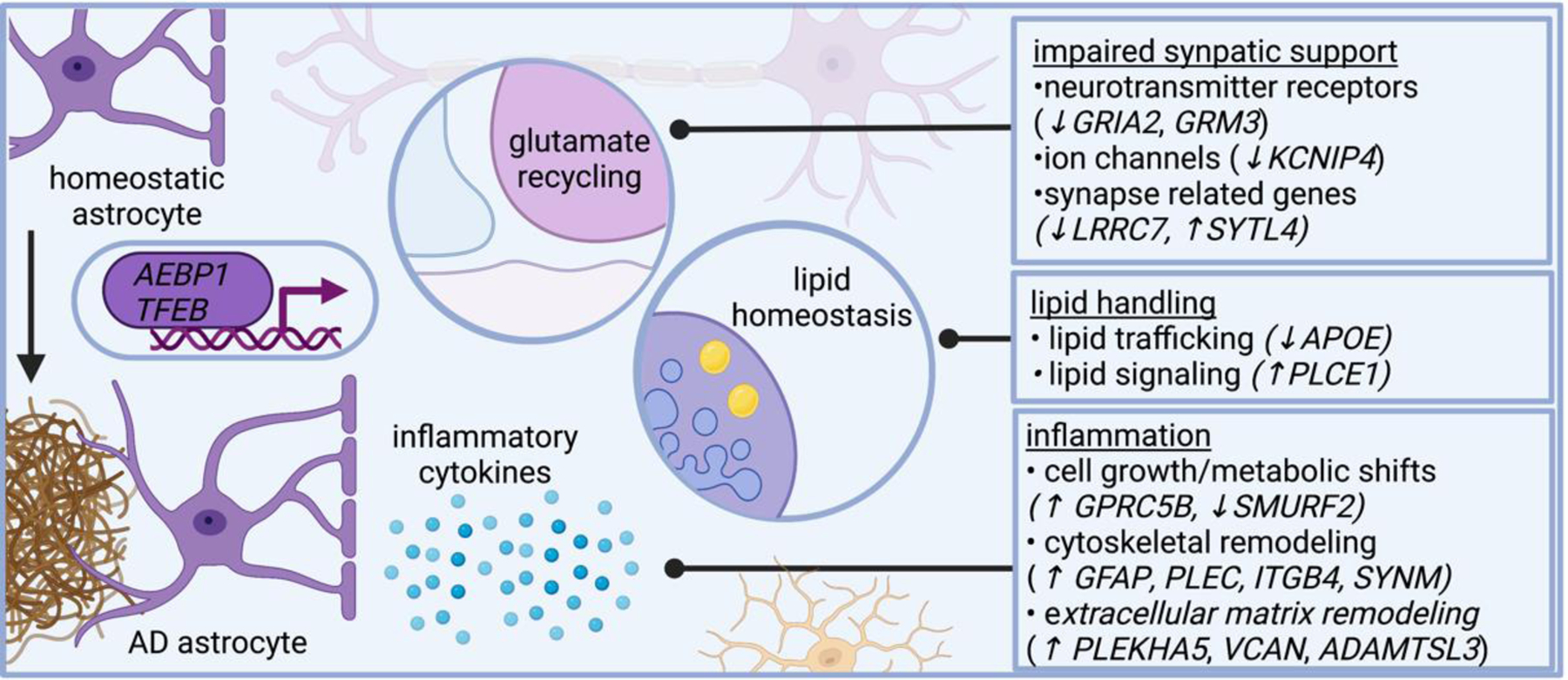
Several lines of evidence suggest astrocytes in AD become inflammatory and impair neural circuit function, including plaque-associated barriers, and modulating lipid-related signaling networks. Single cell genomics shed additional insight on these pathways and reveals astrocytes in AD modulate genes related to neurotransmitter recycling, inflammatory response, and lipid metabolism. AD dysregulates astrocytic genes involved in neurotransmitter receptors (such as GRIA2 and GRM3, which encode subunits of glutamate receptors), ion channels (such as KCNIP4, which encodes a protein that interacts with voltage-gated potassium channels), and even genes involved in synapses (such as LRRC7, which encodes a component of the post synaptic density of excitatory synapses, and SYTL4, which encodes a synaptotagmin). AD astrocytes also modulate genes involved in lipid metabolism, including APOE and PLCE1, which encodes a phospholipase. Several astrocytic genes differentially expressed in AD relate to cytoskeletal remodeling, including GFAP (which encodes an intermediate filament), PLEC (which encodes plectin, a protein that interacts with intermediate filaments), SYNM (which encodes another intermediate filament), and ITGB4 (which encodes an integrin). AD astrocytes modify genes involved in cell growth, such as SMURF2 (a member of the SMAD family important for cell growth). Collectively, these transcriptional changes highlight signaling pathways altered in AD astrocytes.
