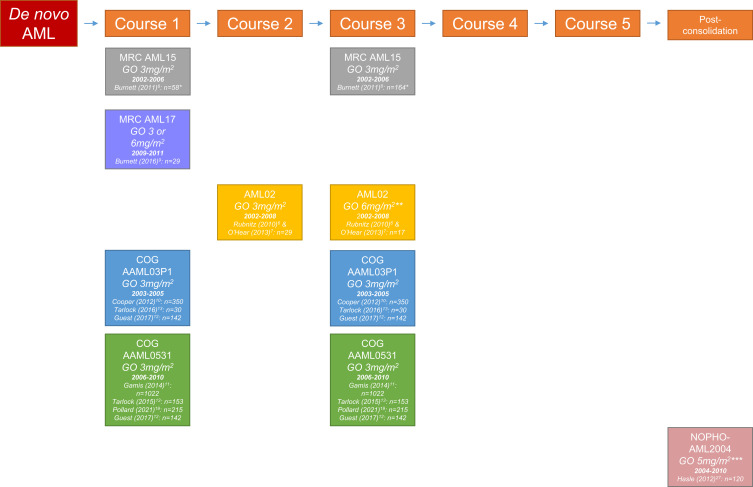
Figure 3.
Overview of study groups assessing the effect of gemtuzumab ozogamicin during different time periods in treatment of pediatric acute myeloid leukemia.
Notes: The number of patients concerns pediatric patients who were randomized to either receive GO or not. *Patients 0–14 years. **Initially, the combination of ADE + GO was used only in patients with >25% bone marrow blasts after induction 1. Patients with an MRD level >0.1% after induction 2 were given GO at a dose of 6 mg/m2 as induction 3. The protocol was amended in 2005 to give ADE + GO as induction 2 to patients with an MRD level >1% after induction 1, and thereby eliminate the use of single-agent GO as induction 3. ***As post-consolidation therapy, repeated after 3 weeks.
Abbreviations: ADE, Ara-C + daunorubicin + etoposide; AML, acute myeloid leukemia; COG, Children’s Oncology Group; GO, gemtuzumab ozogamicin; MRC, Medical Research Council; NOPHO, Nordic Society of Paediatric Haematology and Oncology.