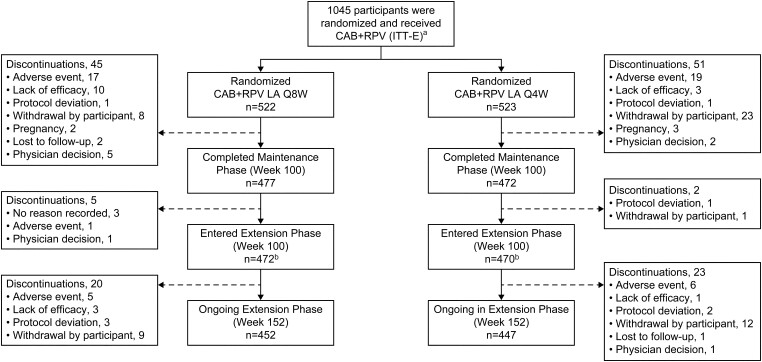
Figure 1.
Participant disposition. No study withdrawals were due to COVID-19 adverse events; however, 2 participants withdrew for COVID-19–related reasons (travel restrictions, n = 1; participant did not want to return to the clinic due to the pandemic, n = 1). aA total of 1049 participants were randomized. However, 4 participants did not receive the study drug and therefore were not part of the ITT-E population. bSeven participants completed the maintenance phase but did not enter the extension phase (Q8W, n = 5; Q4W, n = 2). Abbreviations: CAB, cabotegravir; COVID-19, coronavirus disease 2019; ITT-E, intention-to-treat exposed; LA, long-acting; Q4W, every 4 weeks; Q8W, every 8 weeks; RPV, rilpivirine.