Abstract
Background
We report the yield of targeted universal tuberculosis (TB) testing of clinic attendees in high-risk groups.
Methods
Clinic attendees in primary healthcare facilities in South Africa with one of the following risk factors underwent sputum testing for TB: human immunodeficiency virus (HIV), contact with a TB patient in the past year, and having had TB in the past 2 years. A single sample was collected for Xpert-Ultra (Xpert) and culture. We report the proportion positive for Mycobacterium tuberculosis. Data were analyzed descriptively. The unadjusted clinical and demographic factors’ relative risk of TB detected by culture or Xpert were calculated and concordance between Xpert and culture is described.
Results
A total of 30 513 participants had a TB test result. Median age was 39 years, and 11 553 (38%) were men. The majority (n = 21734, 71%) had HIV, 12 492 (41%) reported close contact with a TB patient, and 1573 (5%) reported prior TB. Overall, 8.3% were positive for M. tuberculosis by culture and/or Xpert compared with 6.0% with trace-positive results excluded. In asymptomatic participants, the yield was 6.7% and 10.1% in symptomatic participants (with trace-positives excluded). Only 10% of trace-positive results were culture-positive. We found that 55% of clinic attendees with a sputum result positive for M. tuberculosis did not have a positive TB symptom screen.
Conclusions
A high proportion of clinic attendees with specific risk factors (HIV, close TB contact, history of TB) test positive for M. tuberculosis when universal testing is implemented.
Keywords: tuberculosis, active case-finding, Xpert, subclinical tuberculosis
Clinic attendees in South Africa at high risk for tuberculosis (TB) living with human immunodeficiency virus, having close contact with a TB patient, or with a prior history of TB underwent universal sputum testing irrespective of the presence of TB symptoms. A high proportion (6%) had a positive test for TB.
The World Health Organization (WHO) estimates that 40% of people with active tuberculosis (TB), more than 4 million people, are not diagnosed or started on TB treatment [1]. Dubbed the “missing cases,” identifying and treating this group are central to the WHO End TB Strategy [2]. South Africa, with the second highest annual incidence of TB in the world [1], has an estimated 150 000 cases of untreated TB per year, accounting for 40% of the country’s total TB burden [3, 4]. Global TB control strategies have focused primarily on passive identification of symptomatic individuals who present to healthcare facilities. However, this symptom-directed approach is inadequate for detecting the majority of people with TB [5–7]. The WHO 4-question symptom screen (cough, fever, weight loss, and night sweats) misses up to half the TB cases among people with human immunodeficiency virus (HIV) on antiretroviral therapy (ART) [8] and 70% of pregnant women with HIV and TB [9–11]. These cases are missed due to both the poor reliability of symptom screening in facilities [12–14] and to a subset of people with subclinical TB (ie, people who have no symptoms or minimal symptoms) [15–25].
Targeted Universal Testing for TB (TUTT) was a cluster randomized trial that compared standard-of-care symptom-directed testing for pulmonary TB to universal testing in high-risk groups in 62 primary healthcare clinics in South Africa. In TUTT, we targeted clinic attendees with HIV, those who self-reported close contact with a TB patient, and those with a history of TB in the preceding 2 years [26–30]. The main findings of the TUTT trial have been reported elsewhere [31]. In this study, we report on the yield of testing and the performance of Xpert-Ultra Mycobacterium tuberculosis/rifampin (Xpert) relative to liquid culture in each of the 3 high-risk groups in the TUTT intervention arm [31].
METHODS
Setting and Study Design
Sixty-two clinics in 3 South African provinces (Gauteng, KwaZulu Natal, and the Western Cape) were selected for randomization in the trial if they diagnosed an average of ≥10 patients/month with TB. Three additional facilities were added to the intervention arm post hoc due to facility closures or other competing research in the same facilities. The 33 intervention clinics are included in this analysis.
Study Procedures
The intervention period was from March 2019 to March 2020 and halted 1 month prior to the planned study end date due to the South Africa coronavirus disease 2019 lockdown. Study team members introduced the study to clinic attendees in waiting areas, inviting them to participate. Additionally, clinic nurses informed potential participants of the study. Eligible participants provided written informed consent. A brief questionnaire was used to elicit a standard WHO TB symptom screen with sociodemographic and clinical variables. We did not ask clinic attendees their reasons for clinic attendance. People attending the clinic for nonclinical reasons, including accompanying others or collecting medication, were eligible for participation. All participants were requested to provide 1 spot, spontaneously expectorated sputum. If unable to produce sputum, they were asked to give a forced cough effort, spit whatever was in their mouth, and repeat. Routine specimen transport was used to deliver specimens to the nearest public sector laboratory with mycobacterial culture capacity.
Laboratory Testing
Testing was performed at 4 public sector National Health Laboratory Service laboratories. Specimens were decontaminated with N-acetyl-L-cystine and sodium hydroxide and then centrifuged. The resulting pellet was resuspended and split for Xpert (Cepheid, Sunnyvale, CA) and for liquid mycobacterial culture testing using the Mycobacterial Growth Indicator Tube (MGIT) automated Bactec 960 instruments (Becton Dickinson, Franklin Lakes, NJ). Species identification of culture-positive specimens was performed using 1 of the following: MPT64 antigen, GenoType MTBDRplus, or GenoType Mycobacterium CM line probe assays (Hain Lifesciences, Germany). Results of microbiological tests were made available to clinics through routine reporting systems. Positive results for M. tuberculosis were also sent to study staff who notified clinics.
Classification of Xpert Results
Xpert results were categorized as positive for M. tuberculosis, negative, or trace. Trace is a semiquantitative category that corresponds to the detection of a very low bacillary load. Because of concerns regarding the specificity of Xpert trace results [32, 33], the interpretation varies according to the clinical scenario. In South Africa, the guideline is to await confirmatory TB culture prior to treatment except in people with HIV and no prior history of TB in whom Xpert trace results are sufficient for treatment [34]. We classified Xpert results as follows: total positive, including trace, all results where M. tuberculosis was detected by Xpert, including trace-positive; trace reclassified, Xpert reclassified as TB-negative in participants with a prior history of TB; and trace excluded, all trace-positive Xpert results were reclassified as TB-negative
Data Analysis
Participants were excluded from the yield analysis if they did not produce a specimen, testing was not performed due to specimen loss or leak, or there was culture contamination or growth of nontuberculous mycobacteria.
Descriptive statistics are presented using counts, proportions, and medians with interquartile ranges (IQRs). We further stratified results by province, self-reported TB symptoms, CD4 count, and HIV treatment status. We report the number needed to be tested (NNT) to identify 1 person with a positive test for M. tuberculosis. In those participants whose specimen provided both Xpert and culture results, we report concordance between the 2 assays. Participants with more than 1 targeted risk factor were included in each of their group analyses. We used log binomial regression and adjusted for clustering by clinic to calculate the relative risk (RR) of having a positive TB test by patient and clinical characteristics.
Approval for this study was obtained from the University of the Witwatersrand Human Research Ethics Committee and by research committees of the 3 Provincial Departments of Health. Written informed consent was required for study participation.
RESULTS
Participant Characteristics
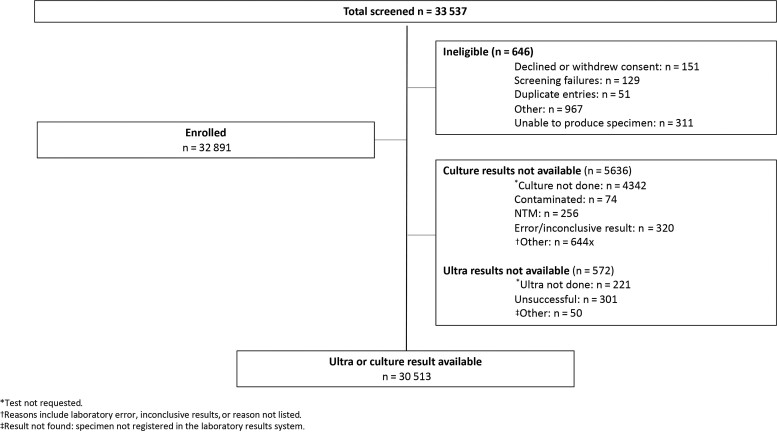
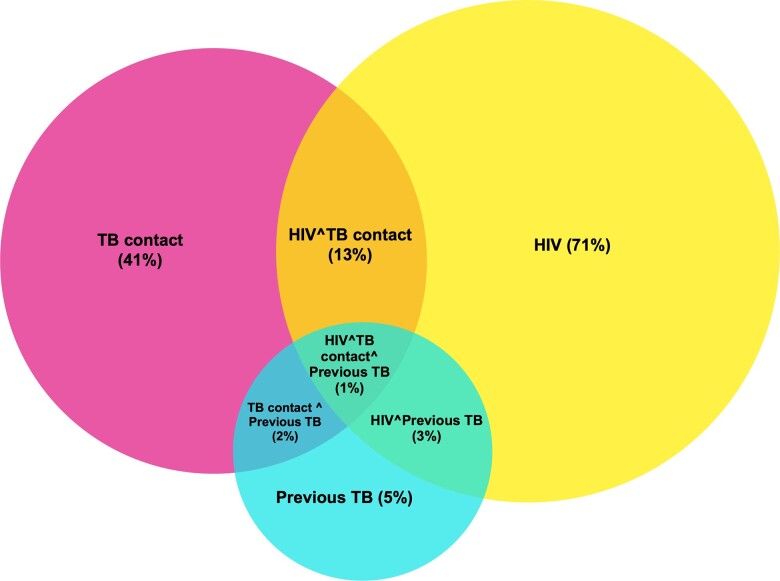
A total of 33 537 participants were screened and consented, and 646 were ineligible (Figure 1). Of the 32 891 enrolled participants, 30 513 (93%) had either or both an Xpert or MGIT result available and were included in this analysis. The median age was 39 years (IQR, 30–46), and 38% of participants were men (Table 1). Of the 3 targeted risk factors, 71% (n = 21 734 of 30 510) of participants had HIV, 41% (n = 12 492 of 30 496) reported a recent close contact with a TB patient, and 5% (n = 1573 of 30 476) had TB in the preceding 2 years (Figure 2). Among participants with HIV in whom ART treatment status was recorded (n = 8510), 87% reported being on ART, and the median duration on treatment was 3.2 years (IQR, 1.1–6.0). The most recent CD4 count was recorded in 40% of study participants with HIV. The median CD4 value was 422 cells/mm3 (IQR, 248–613). Overall, 27% (95% confidence interval [CI]: 26%–27%) of participants reported at least 1 TB symptom (cough, loss of weight, fever or night sweats). Supplementary Table 1 provides a description of participant characteristics with nonoverlapping risk factors (HIV, prior TB and no HIV, and household contact without HIV or prior TB).
Figure 1.
Participant characteristics. Abbreviation: NTM, non-tuberculous mycobacteria.
Table 1.
Demographic and Clinical Characteristics of Study Participants Enrolled at Intervention Clinics of a Cluster Randomized Trial of Targeted Universal Testing for Tuberculosis in High-Risk Groups
| Characteristic | Entire Cohorta (n = 30 513) | HIVa (n = 21 734) | TB Contacta (n = 12 492) | Prior TBa (n = 1573) |
|---|---|---|---|---|
| Age, median (IQR), y | 39 (30–46) | 39 (31–46) | 39 (27–49) | 40 (30–48) |
| Gender, no. (%) | ||||
| ȃMissing | 26 (0) | 15 (0) | 15 (0) | 3 (0) |
| ȃFemale | 18 934 (62) | 14 124 (65) | 7359 (59) | 757 (48) |
| ȃMale | 11 553 (38) | 7595 (35) | 5118 (41) | 813 (52) |
| Symptom status, no. (%) | ||||
| ȃMissing | 41 (0) | 36 (0) | 7 (0) | 3 (0) |
| ȃAsymptomatic | 22 255 (73) | 16 970 (78) | 7796 (62) | 868 (55) |
| ȃSymptomatic | 8217 (27) | 4728 (22) | 4689 (38) | 702 (45) |
| Human immunodeficiency virus status, no. (%) | ||||
| ȃMissing | 587 (2) | … | 568 (5) | 41 (2) |
| ȃNegative | 8192 (29) | … | 7905 (63) | 531 (34) |
| ȃPositive | 21 734 (71) | … | 4019 (32) | 1001 (64) |
| ȃCD4 count available,b no. (%) | 8700 (40) | … | 1618 (40) | 489 (49) |
| ȃCD4 count, median (IQR), cells/mm3 | 422 (248–613) | … | 472 (298–674) | 294 (147–523) |
| ȃART status knownb | 8510/21 734 (39%) | … | 1529/4019 (38%) | 327/1001 (33%) |
| ȃOn ART at enrollment (%) | 7421/8510 (87%) | … | 1132/1529 (74%) | 279/327 (85%) |
| TB contact, no. (%) | ||||
| ȃMissing | 17 (0) | 15 (0) | … | 4 (0) |
| ȃNo | 18 004 (59) | 17 700 (81) | … | 1076 (68) |
| ȃYes | 12 492 (41) | 4019 (19) | … | 493 (31) |
| Prior TB, no. (%) | ||||
| ȃMissing | 37 (0) | 25 (0) | 12 (0) | … |
| ȃNo | 28 903 (95) | 20 708 (95) | 11 987 (96) | … |
| ȃYes | 1573 (5) | 1001 (5) | 493 (4) | … |
| ȃCompleted treatment | 599 (38) | … | … | … |
| ȃLong-term follow-up | 38 (2) | … | … | … |
| ȃOutcome unknown | 936 (60) | … | … | … |
| Time since TB treatment stopped, no. (%) | ||||
| ȃMissing | 68 (4) | … | … | … |
| ȃ<1 y | 505 (32) | … | … | … |
| ȃ1–2 y | 484 (31) | … | … | … |
| ȃ2–5 y | 448 (28) | … | … | … |
| >5 y | 68 (4) | … | … | … |
| Province | ||||
| ȃGauteng, no. (%) | 6593 (22) | 5816 (27) | 877 (7) | 111 (7) |
| ȃKwazulu-Natal, no. (%) | 14 381 (47) | 9480 (44) | 7586 (61) | 1007 (64) |
| ȃWestern Cape, no. (%) | 9539 (31) | 6438 (30) | 4029 (32) | 455 (29) |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; TB, tuberculosis.
Note that the row totals and percentages do not total 100% in all cases as the risk factor groups are not mutually exclusive.
The protocol was amended to include these questions partway through the study, and these data points were collected after recruitment was underway.
Figure 2.
Patient overlap. Abbreviations: HIV, human immunodeficiency virus; TB, tuberculosis.
Yield by Risk Factor
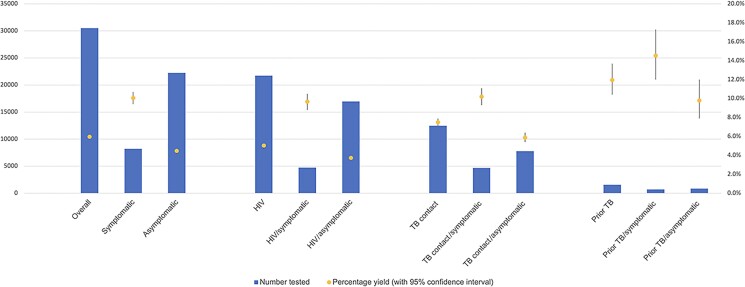
Overall, 8.3% (95% CI: 7.9%–8.6%) of participants had a positive test for M. tuberculosis by culture and/or Xpert; 8.1% (95% CI: 7.8%–8.4%) with trace-positive results were reclassified as negative in those with prior TB and 6.0% with trace-positives excluded (95% CI: 5.7%–6.2%; Table 2A, Figure 3). The overall yield in people with HIV was 7.4% (95% CI: 7.1%–7.8%), 7.2% (95% CI: 6.9%–7.6%) and 5.0% (95% CI: 4.7%–5.3%) with trace-positive results reclassified and with trace-positive results excluded, respectively. Similarly, among people with a close TB contact, yield was 9.8% overall (95% CI: 9.2%–10.3%), 9.6% (95% CI: 9.1%–10.2%) and 7.5% (95% CI: 7.0%–8.0%) with trace-positive results reclassified and with trace-positive results excluded, respectively. The highest yield was among participants with a prior history of TB in the preceding 2 years: 16.3% overall (95% CI: 14.5%–18.2%) and 12.0% with Xpert trace-positives excluded (95% CI: 10.3%–13.6%).
Table 2.
Results and Number Needed to Be Tested of Xpert and Liquid Mycobacterial Culture Tests in Participants Recruited in the Targeted Universal Testing for Tuberculosis Trial Intervention Arm Clinics
| A. Yield of Xpert and Culture in the 3 Targeted risk Groups | ||||
|---|---|---|---|---|
| Test Type | Overall (n = 30 513), n/yield (%) | Human Immunodeficiency Virus (n = 21 734), n/yield (%) |
TB Contact (n = 12 492), n/yield (%) | Prior TB (n = 1573), n/yield (%) |
| Xpert | ||||
| ȃResult available | 29 941 (98.1) | 21 345 (98.2) | 12 239 (98.0) | 1539 (97.8) |
| ȃTotal positive, trace included | 2327 (7.8) | 1476 (6.9) | 1131 (9.2) | 242 (15.7) |
| ȃTotal positive, trace reclassifieda,b | 2253 (7.5) | 1426 (6.7) | 1111 (9.1) | N/A |
| ȃTotal positive, trace excludedb | 1552 (5.2) | 898 (4.2) | 821 (6.7) | 171 (11.1) |
| Culture | ||||
| ȃResult available | 24 877 (81.5) | 18 103 (83.3) | 9633 (77.1) | 1130 (71.8) |
| ȃTotal positive | 1064 (4.3) | 632 (3.5) | 557 (5.8) | 85 (7.5) |
| Xpert and/or culture positive (trace included) |
2531 (8.3) | 1616 (7.4) | 1219 (9.8) | 257 (16.3) |
| NNT | 12 | 13 | 10 | 6 |
| Xpert and/or culture positive (trace reclassified)b |
2460 (8.1) | 1568 (7.2) | 1200 (9.6) | N/A |
| NNT | 12 | 14 | 10 | N/A |
| Xpert and/or culture positive (trace excluded)c |
1823 (6.0) | 1094 (5.0) | 936 (7.5) | 188 (12.0) |
| NNT | 17 | 20 | 13 | 8 |
| B. Yield of Xpert and Culture in Asymptomatic Participants in the 3 Targeted Risk Groups | ||||
|---|---|---|---|---|
| Test Type | Overall (n = 22 255), n/yield (%) | HIV (n = 16 970), n/yield (%) | TB contact (n = 7796), n/yield (%) | Prior TB (n = 868), n/yield (%) |
| Xpert | ||||
| ȃResult available | 21 829 (98) | 16 663 (98) | 7624 (98) | 853 (98) |
| ȃTotal positive, trace included | 1350 (6.2) | 917 (5.5) | 575 (7.5) | 115 (13.5) |
| ȃTotal positive, trace reclassifiedb | … | … | … | … |
| ȃTotal positive, trace excludedb | 812 (3.7) | 506 (3.0) | 385 (5.0) | 76 (8.9) |
| Culture | … | … | … | 38 (5.4%) |
| ȃResult available | 19 345 (87) | 14 855 (89) | 6539 (84) | 708 (82) |
| ȃTotal positive | 595 (3.1) | 384 (2.6) | 270 (4.1) | 38 (5.4) |
| Xpert and/or culture positive (trace included) |
1494 (6.7) | 1015 (6.0) | 635 (8.1) | 122 (14.1) |
| NNT | 15 | 17 | 12 | 7 |
| Xpert and/or culture positive (trace reclassified)b |
1455 (6.5) | 990 (5.8) | 623 (8) | N/A |
| NNT | 15 | 17 | 13 | N/A |
| Xpert and/or culture positive (trace excluded)c |
994 (4.5) | 635 (3.7) | 458 (5.9) | 85 (9.8) |
| NNT | 22 | 27 | 17 | 10 |
Abbreviations: N/A, not applicable; NNT, number needed to be tested; TB, tuberculosis.
Note denominator change for trace reclassification based on availability of prior TB history: n = 29 938 for the entire cohort, n = 19 917 for people with human immunodeficiency virus, and n = 12 238 for TB contacts.
Trace-reclassified participants who tested TB-positive based on a trace-positive Xpert result were reclassified as TB-negative if they had a history of prior TB.
Trace positive–excluded participants who tested TB-positive based on a trace-positive Xpert result were reclassified as TB-negative.
Figure 3.
Overall patient description. Abbreviations: HIV, human immunodeficiency virus; TB, tuberculosis.
The overall NNT to obtain 1 positive test using culture and Xpert was 12, with all Xpert-positive results inclusive of trace and 17 with trace-positive results excluded. Similarly, in individuals with HIV, NNT were 13 and 20, respectively; in those with a TB contact, NNT was 10 and 13, respectively; and in the group with prior TB, NTT was 6 and 8, respectively.
Yield in Participants Based on Reported Symptom Status
Overall, of participants with a positive TB test (MGIT- and/or Xpert-positive, trace excluded), only 45% (826 of 1820) reported at least 1 symptom of TB. Among participants who were WHO symptom screen–negative, the yield was 6.7% (95% CI: 6.4%–7.0%) by Xpert and/or culture and 6.5% (95% CI: 6.3%–6.9%) with trace-positive results reclassified as negative in those with prior TB and 4.5% (95% CI: 4.2%–4.7%) with trace-positive results excluded (Table 2B). The overall asymptomatic NNT was 22 vs 15; 27 vs 17 in people with HIV; 17 vs 12 in TB contacts; and 10 vs 7 in those with a prior history of TB depending on the inclusion of trace results. The yield in symptomatic participants is described in Supplementary Table 2. However, there was significant variability in the frequency of symptom screen positivity by interviewer, ranging from 0% to 85% (median, 27%; IQR, 5%–52%; Supplementary Figure 2). Furthermore, in the first 3 months of the study (May 2019–July 2019), a much higher proportion of interviewers reported symptom positivity among participants (median symptom positivity rate, 57% per interviewer [IQR, 42%–72%] vs in the last 3 months of the study (January 2020–March 2020; median, 3% symptom positivity; IQR, 0.5%–22%).
Variability in Yield by Province and Facility
The yield of testing varied considerably between provinces and facilities. The yield was 2.0% (95% CI: 1.7%–2.4%) in Gauteng, 7.1% (95% CI: 6.5%–7.6%) in KwaZulu Natal, and 7.0% (95% CI: 6.5%–7.5%) in the Western Cape (trace-positives excluded). Moreover, individual clinics had markedly different yields within the same province (Supplementary Table 3).
Yield in HIV by ART Status, CD4 Strata, and Presence of Reported Symptoms
In participants with HIV on ART, 4.0% (95% CI: 3.5%–4.4%) were positive for M. tuberculosis (trace-positives excluded; Supplementary Figure 1A), whereas in those not on ART, 12.2% (95% CI: 10.4%–14.1%) had a positive test. The yield was highest (5.1%; 95% CI: 4.0%–6.3%) in those with CD4 <200 cells/mm3, decreasing with increasing CD4 count to 3% (95% CI: 2.4%–3.6%) in participants with CD4 >500 cells/mm3 (Supplementary Figure 1B). Most people with HIV and a positive test for M. tuberculosis did not report TB symptoms; only 19% (n = 57 of 293; 95% CI: 15%–24%) of people on ART with TB and 39% (n = 51 of 130; 95% CI: 31%–48%) of those not on ART with TB reported at least 1 symptom of TB.
Individual-level Risk Factors for TB
In an unadjusted log binomial regression analysis adjusted for clustering by clinic (Table 3), men had higher relative risk of M. tuberculosis than women (RR, 2.2; 95% CI: 2.1–2.5). Although fewer than half of the identified TB cases (45%) occurred in people in whom at least 1 symptom of TB was recorded, symptomatic patients were at increased risk of having a positive TB test (RR, 2.3; 95% CI: 1.7–2.9). People with HIV accounted for 60% of the identified TB cases and had a lower risk of having a positive TB test compared with those who were HIV-seronegative and had at least 1 of the other 2 study risk factors (RR, 0.6; 95% CI: .5–.8). Adults with HIV and not on ART had a 3-fold higher risk of testing positive for M. tuberculosis (RR, 3.1; 95% CI: 2.0–4.7) compared with those on ART.
Table 3.
Association of Covariates With Positive Tuberculosis Test for Mycobacterium tuberculosis
| Characteristic | Entire Cohort (n = 30 513) | a Mycobacterium tuberculosis Positive (n = 1823) | Relative Risk (95% Confidence Interval) |
|---|---|---|---|
| Age category (n = 305 100), y | |||
| ȃ18–29 | 7524 | 557 (7.4%) | 1.0 (reference) |
| ȃ30–39 | 10 020 | 640 (6.4%) | .9 (.8–1) |
| ȃ40–49 | 7132 | 376 (5.3%) | .7 (.6–.9) |
| ȃ50+ | 5834 | 250 (4.3%) | .6 (.4–.8) |
| Sex (n = 30 487) | |||
| ȃFemale | 18 934 | 769 (4.1) | 1.0 (reference) |
| ȃMale | 1153 | 1052 (9.1%) | 2.2 (2.1–2.5) |
| Symptom status (n = 30 472) | |||
| ȃNo symptoms reported | 22 255 | 994 (4.5%) | 1.0 (reference) |
| ȃSymptomatic | 8217 | 826 (10.1%) | 2.3 (1.7–2.9) |
| HIV status (n = 30 510) | |||
| ȃNo HIV | 8192 | 665 (8.1%) | 1.0 (reference) |
| ȃHIV | 21 734 | 1094 (5.0%) | .6 (.5–.8) |
| ȃUnknown | 587 | 64 (11.0%) | 1.3 (1.1–1.7) |
| ART status (n = 8510) | |||
| ȃOn ART at enrollment | 7421 | 294 (4.0%) | 1.0 (reference) |
| ȃNot on ART at enrollment | 1089 | 130 (11.9%) | 3.1 (2.0–4.7) |
| TB contact (n = 30 496) | |||
| ȃNo | 18 004 | 887 (5.0%) | 1.0 (reference) |
| ȃYes | 12 492 | 936 (7.5%) | 1.5 (1.2–1.9) |
| Prior TB (n = 30 476) | |||
| ȃNo | 28 903 | 1634 (5.7%) | 1.0 (reference) |
| ȃYes | 1573 | 188 (12.0%) | 2.1 (1.7–2.7) |
| Province (n = 30 513) | |||
| ȃGauteng | 6593 | 131 (2.0%) | 1.0 (reference) |
| ȃKwaZulu Natal | 14 381 | 1027 (7.1%) | 3.6 (2.5–5.2) |
| ȃWestern Cape | 9539 | 665 (7.0%) | 3.5 (2.5–4.8) |
At least 1 laboratory result (Xpert and/or Mycobacterial Growth Indicator Tube culture) positive for Mycobacterium tuberculosis (Xpert trace-positive results excluded). Data are unadjusted relative risk (95% confidence interval). Expert trace results excluded.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; TB, tuberculosis.
Concordance Between Xpert and Culture
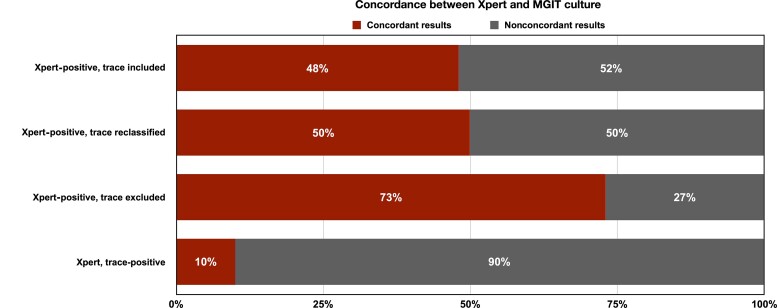
The concordance between Xpert results and culture results was poor, and half of all positive Xpert tests in the study (48%; 860 of 1775; 95% CI: 46%–51%) were culture-negative (Figure 4, Supplementary Table 4). When Xpert trace-positive results were classified as negative, the concordance improved to 73% (793 of 1093; 95% CI: 70%–75%). Reclassifying trace-positive results as negative in those with prior TB did not alter concordance between Xpert and culture significantly given the limited number of people with prior TB. The concordance between trace-positive results and culture was extremely poor, and only 10% were culture-positive for M. tuberculosis (67 of 682; 95% CI; 8%–12%).
Figure 4.
Concordance between Xpert and MGIT culture. Abbreviation: MGIT, Mycobacterial Growth Indicator Tube.
DISCUSSION
Our study shows that the yield of universal testing for pulmonary TB in clinic attendees at high risk of TB is high when all are requested to provide a sputum specimen, irrespective of the outcome of symptom screening. Indeed, in this study, between 6% and 8% had an Xpert or culture result positive for M. tuberculosis depending on the interpretation of trace-positive results. We further show that the yield of testing was high even in those in whom no history of TB symptoms was elicited; 4.5% had a positive test for M. tuberculosis. Additionally, Xpert had poor concordance with MGIT liquid culture in this population, with only half of all Xpert-positive results being culture positive. Finally, there was substantial regional and facility variability in the yield of testing, ranging from 1% to 13%, suggesting that additional targeting by province and clinic could further refine the targeted testing strategy we report here [35].
Although the yield was much higher in people who reported 1 or more TB symptoms in the WHO symptom screen, a substantial proportion of bacteriologically confirmed cases would be missed by ignoring high-risk groups in whom symptoms are not elicited by healthcare providers. Overall, no history of TB symptoms was elicited in 55% of the positive TB cases in this study [36]. Among people with HIV, our finding that 3.7% of clinic attendees had TB but did not report TB symptoms is consistent with prior data from the region [15, 37, 38]. The proportion of positive TB cases who were symptom screen–negative was higher among those on ART than those not on ART, which is also consistent with findings from a large meta-analysis of the sensitivity of the WHO symptom screen in people with HIV [8]. It remains unknown if these participants were truly asymptomatic or if this was the result of the poor reliability of symptom screening. The variability in positive symptom screen rates among interviewers and across the duration of the trial suggests that symptom screening was not consistently administered. This lends further support that high-quality, consistent TB symptom screening is challenging to implement at scale [12–14] and that nonsymptom-based screening approaches are required to identify TB in high-risk groups in healthcare facilities [39].
Of the 3 targeted risk groups, the yield of testing was highest among those with a prior history of TB; 12% had detectable TB, supporting calls for intensive follow-up of people who recently completed TB treatment [28–30, 40]. However, they represented a small fraction (5%) of all participants in this study and only 10% of all diagnosed TB cases, making this a challenging population to identify in primary healthcare settings. The high rate of HIV coinfection in this group (64%) suggests that most of the TB cases could have been identified by targeting people within the HIV treatment program. Although there was a 3-fold higher risk of TB in those not on ART compared with those who initiated ART, 75% of TB cases occurred in people on ART, suggesting that a focus of universal TB testing on adults not yet on ART [41] would miss most of the prevalent TB in this risk group. Last, our data demonstrate that the targeted testing of TB contacts attending clinics could offer a potentially cost-effective alternative to community and home-based screening of TB contacts as the numbers of TB contacts were readily identified in study clinics.
We found that male clinic attendees were more than twice as likely to have TB than female clinic attendees, which accords with the epidemiology of TB in sub-Saharan Africa [3, 41]. The lower participation of men in our study mirrors the lower engagement of men in primary healthcare and HIV services in the region [41–43]. However, this study demonstrates that a clinic-based intervention can be an effective option for finding prevalent TB in men.
The most concerning finding of our study was the poor concordance between Xpert and culture. Crucially, this finding was not limited to trace-positive results. In our study, only 48% of Xpert-positive results were culture-positive. Moreover, this only improved to 73% when trace-positive results were excluded (only 10% of trace-positive results were MGIT-positive). This is comparable to the rate of concordance between Xpert and culture seen in other studies where people were tested irrespective of symptoms (eg, prevalence surveys; high-risk groups such as miners, people with HIV, household contacts) [44, 45]. Most notably, in the South African National Prevalence Survey in which people were tested on the basis of symptoms or an abnormal chest X-ray, only 65% of positive Xpert results (including trace) were culture-positive [44]. These findings contrast with the performance of Xpert in presumptive TB cases (ie, people with symptoms), where 90% of positive Xpert results [32] and 30%–50% of trace-positive results were culture-positive [35, 46]. There are multiple possible explanations for the low Xpert vs culture concordance we report. First, MGIT is an imperfect gold standard and may miss some true-positive cases [47, 48]. Also, by splitting specimens and decreasing the mycobacterial burden in each sample, the sensitivity of culture for detecting TB may have been reduced and contributed to the elevated rate of discordance seen in this study. Furthermore, we know that Xpert can be positive in people with prior treated TB who have mycobacterial DNA but no replicating bacteria, and our study population was enriched for people with prior TB. Given that the reported rate of prior TB in people with HIV ranges from 8% to 25% in the region [15, 49–51], this is going to be a significant challenge to implementation of universal testing for TB using Xpert in people with HIV in ART facilities. Further work to evaluate this population prospectively with serial sampling, chest imaging, and longitudinal follow-up is critical to understanding the clinical implications of molecular test–positive, culture-negative results, especially in people with no prior history of TB. It is not known if this is a group at risk of progression to clinical TB disease, whether treatment is indicated, and if they pose a transmission risk.
This study has several important limitations. Study participants provided informed consent and underwent an interview. This could have resulted in sampling bias as people willing to participate in a study may not accurately reflect clinic populations. In order to increase enrollment and minimize bias against the intervention in the primary outcome of the study, which was to determine if risk factor–based screening could increase the number of TB cases in a cluster randomized trial design [31], the interview and data collection process was kept short. Given the scale of the study (>30 000 participants in the intervention facilities), a long interview would have resulted in missed opportunities for recruitment. Initially, we collected very few variables and did not include history of ART treatment, CD4 count, and outcome of prior TB treatment. The protocol was amended partway through the study, and these data points were collected after recruitment was underway. A second limitation is that the rate of prior TB identified in our study was lower than anticipated. Recent studies that recruited people with HIV from primary healthcare facilities in South Africa have reported rates of prior TB ranging from 8% to 25% [15, 49–51]. It is possible that upon identifying 1 risk factor rendering people eligible for the study (eg, HIV), study recruiters failed to obtain adequate additional history regarding prior history of TB and presence of a contact with TB. Another limitation is the lack of reliability in symptom screening. We found that the rate of positive symptom screens by interviewer was highly variable, ranging from 0% to 85% (median, 27%; IQR, 5%–52%), and dropped over the course of the study (from a median of 57% in the first 3 months to 3% in the last 3 months). We did not report data on TB treatment initiation or treatment success in those diagnosed with TB; this requires additional study, particularly in those who report no symptoms. Finally, we selected the targeted risk groups based on data from South Africa, which limits the generalizability of our findings to other settings where the risk factors for TB may differ [3].
CONCLUSIONS
Our results indicate that case detection strategies based on routine symptom screening of clinic attendees do not identify all adults with pulmonary TB. The targeted universal testing approach described in this study has a high yield for M. tuberculosis and should be part of an expanded testing strategy, although costs and laboratory capacity need to be assessed as barriers to implementation. The high prevalence of pulmonary TB in patients attending primary healthcare clinics presents an important opportunity for early detection of TB that may diminish transmission and also prevent future TB-related morbidity and mortality.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Rebecca H Berhanu, Department of Medicine, Division of Infectious Diseases, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Limakatso Lebina, Perinatal HIV Research Unit (PHRU), University of Witwatersrand, Soweto, South Africa.
Bareng A S Nonyane, Johns Hopkins Bloomberg School of Public Health, Department of International Health, Baltimore, Maryland, USA.
Minja Milovanovic, Perinatal HIV Research Unit (PHRU), University of Witwatersrand, Soweto, South Africa.
Anthony Kinghorn, Perinatal HIV Research Unit (PHRU), University of Witwatersrand, Soweto, South Africa.
Lucy Connell, Right to Care, Johannesburg, South Africa.
Sipho Nyathi, Aquity Innovations, Pretoria, South Africa.
Katherine Young, TB HIV Care, Cape Town, South Africa.
Harry Hausler, TB HIV Care, Cape Town, South Africa; Department of Family Medicine, University of Pretoria, Pretoria, South Africa.
Pren Naidoo, Public Health Management Consultant, Cape Town, South Africa.
Zameer Brey, Bill and Melinda Gates Foundation –South Africa, Johannesburg, South Africa.
Kate Shearer, Department of Medicine, Division of Infectious Diseases, Baltimore, Maryland, USA; Centre for TB Research, Johns Hopkins University, Baltimore, Maryland, USA.
Leisha Genade, Perinatal HIV Research Unit (PHRU), University of Witwatersrand, Soweto, South Africa.
Neil A Martinson, Perinatal HIV Research Unit (PHRU), University of Witwatersrand, Soweto, South Africa; Centre for TB Research, Johns Hopkins University, Baltimore, Maryland, USA.
Notes
Author Contributions. R. H. B. conducted data cleaning and data analysis and drafted the manuscript. N. A. M. and L. L. conceptualized the study and supported data collection, data analysis, and manuscript writing. M. M., A. K., B. A. S. N., and L. G. contributed to study design and supported data collection, data analysis, and review of the manuscript. L. C., S. N., K. Y., and H. H. were involved in study implementation and reviewed the manuscript. Z. B. and P. N. were instrumental in starting the process, contributed to study design, and reviewed the manuscript.
Acknowledgments . We thank the participants who consented to be in the study; the staff of the study clinics; managers in Gauteng, KwaZulu Natal, and the Western Cape Provinces who facilitated our access to these clinics; the South African National Priorities Programme that did the initial analysis of national tuberculosis (TB) result data, which allowed us to select study clinics; and the staff at the National Health Laboratory Service who conducted all sputum testing and willingly provided laboratory results to us and clinics.
Financial support. This work was supported by a grant from the Bill & Melinda Gates Foundation. All laboratory testing was generously paid for by the Government of South Africa. R. H. B. received support from the National Institutes of Health (K08AI150352 and T32AI052074).
References
- 1. World Health Organization . Global tuberculosis report 2022. Geneva, Switzerland: WHO, 2022. [Google Scholar]
- 2. World Health Organization . The End-TB Strategy: global strategy and targets for tuberculosis prevention, care and control after 2015. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 3. World Health Organization . Global tuberculosis report 2020. Geneva, Switzerland: WHO, 2020. [Google Scholar]
- 4. National Department of Health . The first national TB prevalence survey South Africa. Pretoria: South African, NDOH, 2018. [Google Scholar]
- 5. Marks GB, Nguyen NV, Nguyen PTB, et al. . Community-wide screening for tuberculosis in a high-prevalence setting. N Engl J Med 2019; 381:1347–57. [DOI] [PubMed] [Google Scholar]
- 6. Uplekar M, Creswell J, Ottmani SE, Weil D, Sahu S, Lonnroth K. Programmatic approaches to screening for active tuberculosis. Int J Tuberc Lung Dis 2013; 17:1248–56. [DOI] [PubMed] [Google Scholar]
- 7. Golub JE, Dowdy DW. Screening for active tuberculosis: methodological challenges in implementation and evaluation. Int J Tuberc Lung Dis 2013; 17:856–65. [DOI] [PubMed] [Google Scholar]
- 8. Hamada Y, Lujan J, Schenkel K, Ford N, Getahun H. Sensitivity and specificity of WHO's recommended four-symptom screening rule for tuberculosis in people living with HIV: a systematic review and meta-analysis. Lancet HIV 2018; 5:e515–e23. [DOI] [PubMed] [Google Scholar]
- 9. Hoffmann C, Variava E, Rakgokong M, et al. . High prevalence of pulmonary tuberculosis but low sensitivity of symptom screening among HIV-infected pregnant women in South Africa. PLoS One 2013; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gounder C, Wada N, Kensler C, et al. . Active tuberculosis case-finding among pregnant women presenting to antenatal clinics in Soweto, South Africa. J Acquir Immune Defic Syndr 2011; 57:e77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. LaCourse SM, Cranmer LM, Matemo D, et al. . Tuberculosis case finding in HIV-infected pregnant women in Kenya reveals poor performance of symptom screening and rapid diagnostic tests. J Acquir Immune Defic Syndr 2016; 71:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Divala T, Lewis J, Bulterys M, et al. . Missed opportunities for diagnosis and treatment in patients with TB symptoms: a systematic review. Public Health Action 2022; 12:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chihota VN, Ginindza S, McCarthy K, Grant AD, Churchyard G, Fielding K. Missed opportunities for TB investigation in primary care clinics in South Africa: experience from the Xtend trial. PLoS One 2015; 10:e0138149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kweza P, Abraham N, Claassens M, Van Schalkwyk C, Medino-Marino A. Missed pulmonary TB screening opportunities at primary healthcare facilities: an exit study, Eastern Cape Province, South Africa. Int J Infect Dis 2016; 45:34. [Google Scholar]
- 15. Bajema KL, Bassett IV, Coleman SM, et al. . Subclinical tuberculosis among adults with HIV: clinical features and outcomes in a South African cohort. BMC Infect Dis 2019; 19:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drain P, Bajema K, Dowdy D, et al. . Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev 2018; 31:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lawn SD, Kerkhoff AD, Wood R. Progression of subclinical culture-positive tuberculosis to symptomatic disease in HIV-infected individuals. AIDS 2011; 25:2190–1. [DOI] [PubMed] [Google Scholar]
- 18. Swaminathan S, Paramasivan CN, Kumar SR, Mohan V, Venkatesan P. Unrecognised tuberculosis in HIV-infected patients—sputum culture is a useful tool. Int J Tuberc Lung Dis 2004; 8:896–8. [PubMed] [Google Scholar]
- 19. Kendall EA, Shrestha S, Dowdy DW. The epidemiological importance of subclinical tuberculosis. A critical reappraisal. Am J Respir Crit Care Med 2021; 203:168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization . Global tuberculosis report 2019. Geneva, Switzerland: WHO, 2019. [Google Scholar]
- 21. Yates TA, Khan PY, Knight GM, et al. . The transmission of Mycobacterium tuberculosis in high burden settings. Lancet Infect Dis 2016; 16:227–38. [DOI] [PubMed] [Google Scholar]
- 22. Chin DP, Hanson CL. Finding the missing tuberculosis patients. J Infect Dis 2017; 216(suppl_7):S675–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Naidoo P, Theron G, Rangaka MX, et al. . The South African tuberculosis care cascade: estimated losses and methodological challenges. J Infect Dis 2017; 216(suppl_7):S702–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peters JS, Andrews JR, Hatherill M, et al. . Advances in the understanding of Mycobacterium tuberculosis transmission in HIV-endemic settings. Lancet Infect Dis 2019; 19:e65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Auld SC, Shah NS, Cohen T, Martinson NA, Gandhi NR. Where is tuberculosis transmission happening? Insights from the literature, new tools to study transmission and implications for the elimination of tuberculosis. Respirology 2018; 23:807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Panjabi R, Comstock GW, Golub JE. Recurrent tuberculosis and its risk factors—adequately treated patients are still at high risk. Int J Tuberc Lung Dis 2007; 11:828–37. [PubMed] [Google Scholar]
- 27. Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet 2001; 358:1687–93. [DOI] [PubMed] [Google Scholar]
- 28. Marx FM, Floyd S, Ayles H, Godfrey-Faussett P, Beyers N, Cohen T. High burden of prevalent tuberculosis among previously treated people in southern Africa suggests potential for targeted control interventions. Eur Respir J 2016; 48:1227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marx FM, Dunbar R, Enarson DA, et al. . The temporal dynamics of relapse and reinfection tuberculosis after successful treatment: a retrospective cohort study. Clin Infect Dis 2014; 58:1676–83. [DOI] [PubMed] [Google Scholar]
- 30. Marx FM, Yaesoubi R, Menzies NA, et al. . Tuberculosis control interventions targeted to previously treated people in a high-incidence setting: a modelling study. Lancet Global Health 2018; 6:e426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martinson NA, Nonyane BA, Genade LP, et al. . 2022. A cluster randomized trial of systematic targeted universal testing for tuberculosis in primary care clinics of South Africa (the TUTT study). Available at SSRN: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4092970 (accessed 5 January 2022). [DOI] [PMC free article] [PubMed]
- 32. Dorman SE, Schumacher SG, Alland D, et al. . Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis 2018; 18:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kendall E, Schumacher S, Denkinger C, Dowdy D. Estimated clinical impact of the Xpert MTB/RIF Ultra cartridge for diagnosis of pulmonary tuberculosis: a modeling study. PLoS Med 2017; 14:e1002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. World Health Organization . TB Diagnostics operational handbook. Geneva, Switzerland: WHO, 2020. [Google Scholar]
- 35. Ayles H. TB or not TB: comparison of different diagnostic algorithms in the Treats Prevalence surveys. 50th World Conference on Lung Health. Int J Tuberc Lung Dis 2019; 23:S53–4. [Google Scholar]
- 36. Churchyard GJ, Mametja LD, Mvusi L, et al. . Tuberculosis control in South Africa: successes, challenges and recommendations. S Afr Med J 2014; 104(3 Suppl 1):244–8. [DOI] [PubMed] [Google Scholar]
- 37. Brennan A, Maskew M, Larson BA, et al. . Prevalence of TB symptoms, diagnosis and treatment among people living with HIV (plhiv) not on art presenting at outpatient clinics in South Africa and Kenya: baseline results from a clinical trial. BMJ Open 2020; 10:e035794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blanc F-X, Badje AD, Bonnet M, et al. . Systematic or test-guided treatment for tuberculosis in HIV-infected adults. N Engl J Med 2020; 382:2397–410. [DOI] [PubMed] [Google Scholar]
- 39. Frascella B, Richards AS, Sossen B, et al. . Subclinical tuberculosis disease—a review and analysis of prevalence surveys to inform definitions, burden, associations, and screening methodology. Clin Infect Dis 2021; 73:e830–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marx FM, Cohen T, Menzies NA, Salomon JA, Theron G, Yaesoubi R. Cost-effectiveness of post-treatment follow-up examinations and secondary prevention of tuberculosis in a high-incidence setting: a model-based analysis. Lancet Global Health 2020; 8:e1223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chikovore J, Pai M, Horton KC, et al. . Missing men with tuberculosis: the need to address structural influences and implement targeted and multidimensional interventions. BMJ Global Health 2020; 5:e002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Randera-Rees S, Clarence Safari W, Gareta D, Herbst K, Baisley K, Grant AD. Can we find the missing men in clinics? Clinic attendance by sex and HIV status in rural South Africa. Wellcome Open Res 2021; 6:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. UNAIDS . 2022. A snapshot of men and HIV in South Africa. https://www.unaids.org/sites/default/files/snapshot-men-hiv-south-africa_en.Pdf (accessed 13 September 2022).
- 44. Moyo S, Ismail F, Van der Walt M, et al. . Prevalence of bacteriologically confirmed pulmonary tuberculosis in South Africa, 2017–19: a multistage, cluster-based, cross-sectional survey. Lancet Infect Dis 2022; 22:1172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kendall EA, Kitonsa PJ, Nalutaaya A, et al. . The spectrum of tuberculosis disease in an urban Ugandan community and its health facilities. Clin Infect Dis 2021; 72:e1035–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scott L, Da Silva P, Fyve K. Characterisation of South Africa's Xpert MTB/RIF Ultra “trace” laboratory results. [CROI abstract 755]. In special issue: abstracts from the 2020 Conference on Retroviruses and Opportunistic Infections. Top Antivir Med 2020; 28:483. [Google Scholar]
- 47. Gordhan BG, Peters JS, McIvor A, et al. . Detection of differentially culturable tubercle bacteria in sputum using mycobacterial culture filtrates. Sci Rep 2021; 11:6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Parker RA. Implications of tuberculosis sputum culture test sensitivity on accuracy of other diagnostic modalities. Am J Respir Crit Care Med 2019; 199:664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Churchyard G, Cardenas V, Chihota V, et al. . Annual tuberculosis preventive therapy for persons with HIV infection: a randomized trial. Ann Intern Med 2021; 174:1367–76. [DOI] [PubMed] [Google Scholar]
- 50. Mendelsohn SC, Fiore-Gartland A, Awany D, et al. . Clinical predictors of pulmonary tuberculosis among South African adults with HIV. eClinicalMedicine 2022; 45:101328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mendelsohn SC, Mulenga H, Mbandi SK, et al. . Host blood transcriptomic biomarkers of tuberculosis disease in people living with HIV: a systematic review protocol. BMJ Open 2021; 11:e048623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.