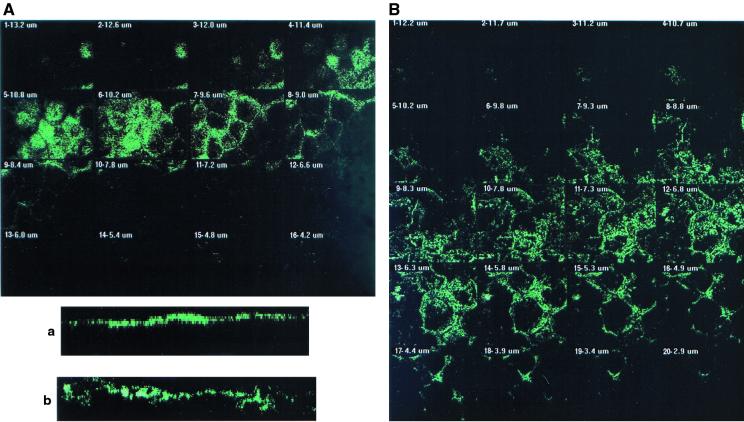
FIG. 2.
CLSM analysis of F-actin immunolabeling in Afa/Dr DAEC C1845-infected Caco-2/TC7 cells. Confluent differentiated Caco-2/TC7 cells were infected apically at 37°C in a 10% CO2–90% air atmosphere for 3 h with C1845 bacteria (108 CFU/well). Cells were fixed with 3.5% paraformaldehyde, washed, permeabilized with Triton X-100, and processed for immunofluorescence labeling as described in Materials and Methods. Fixed and permeabilized cells were processed for direct immunofluorescence labeling of F-actin with fluorescein-labeled phalloidin as described in Materials and Methods. Cells were examined using a confocal laser scanning microscope (model PCM 2000; Diaphot 300 microscope using a 100× Pan Fluor ELSW DM CF160 objective; Nikon). The samples were analyzed by serial optical horizontal sectioning. The section starts at the basal domain of the cells, and the following analysis was conducted until the apical domain was reached. (A and a) Control uninfected cells; (B and b) DAEC C1845-infected cells. (A and B) En face micrographs of the immunolocalization of F-actin obtained in CLSM analysis (horizontal x-y optical sections). In control cells, the majority of F-actin labeling starts on section 3 and afterwards is distributed in six sections (one section every 0.60 μm). In C1845-infected cells, the majority of the F-actin labeling starts on section 5 and afterwards is distributed in 12 sections (one section every 0.50 μm). (a and b) Lateral views of the immunolocalization of F-actin obtained in CLSM analysis (vertical x-z optical section). In control cells, F-actin labeling is localized at the apical domain in a homogenous band. In C1845-infected cells, apical F-actin labeling is dramatically modified, showing a disruption in apical labeling and delocalization of the protein in a nonhomogenous band.