Abstract
Background
Sleep disturbance is common following hospital admission both for COVID-19 and other causes. The clinical associations of this for recovery after hospital admission are poorly understood despite sleep disturbance contributing to morbidity in other scenarios. We aimed to investigate the prevalence and nature of sleep disturbance after discharge following hospital admission for COVID-19 and to assess whether this was associated with dyspnoea.
Methods
CircCOVID was a prospective multicentre cohort substudy designed to investigate the effects of circadian disruption and sleep disturbance on recovery after COVID-19 in a cohort of participants aged 18 years or older, admitted to hospital for COVID-19 in the UK, and discharged between March, 2020, and October, 2021. Participants were recruited from the Post-hospitalisation COVID-19 study (PHOSP-COVID). Follow-up data were collected at two timepoints: an early time point 2–7 months after hospital discharge and a later time point 10–14 months after hospital discharge. Sleep quality was assessed subjectively using the Pittsburgh Sleep Quality Index questionnaire and a numerical rating scale. Sleep quality was also assessed with an accelerometer worn on the wrist (actigraphy) for 14 days. Participants were also clinically phenotyped, including assessment of symptoms (ie, anxiety [Generalised Anxiety Disorder 7-item scale questionnaire], muscle function [SARC-F questionnaire], dyspnoea [Dyspnoea-12 questionnaire] and measurement of lung function), at the early timepoint after discharge. Actigraphy results were also compared to a matched UK Biobank cohort (non-hospitalised individuals and recently hospitalised individuals). Multivariable linear regression was used to define associations of sleep disturbance with the primary outcome of breathlessness and the other clinical symptoms. PHOSP-COVID is registered on the ISRCTN Registry (ISRCTN10980107).
Findings
2320 of 2468 participants in the PHOSP-COVID study attended an early timepoint research visit a median of 5 months (IQR 4–6) following discharge from 83 hospitals in the UK. Data for sleep quality were assessed by subjective measures (the Pittsburgh Sleep Quality Index questionnaire and the numerical rating scale) for 638 participants at the early time point. Sleep quality was also assessed using device-based measures (actigraphy) a median of 7 months (IQR 5–8 months) after discharge from hospital for 729 participants. After discharge from hospital, the majority (396 [62%] of 638) of participants who had been admitted to hospital for COVID-19 reported poor sleep quality in response to the Pittsburgh Sleep Quality Index questionnaire. A comparable proportion (338 [53%] of 638) of participants felt their sleep quality had deteriorated following discharge after COVID-19 admission, as assessed by the numerical rating scale. Device-based measurements were compared to an age-matched, sex-matched, BMI-matched, and time from discharge-matched UK Biobank cohort who had recently been admitted to hospital. Compared to the recently hospitalised matched UK Biobank cohort, participants in our study slept on average 65 min (95% CI 59 to 71) longer, had a lower sleep regularity index (–19%; 95% CI –20 to –16), and a lower sleep efficiency (3·83 percentage points; 95% CI 3·40 to 4·26). Similar results were obtained when comparisons were made with the non-hospitalised UK Biobank cohort. Overall sleep quality (unadjusted effect estimate 3·94; 95% CI 2·78 to 5·10), deterioration in sleep quality following hospital admission (3·00; 1·82 to 4·28), and sleep regularity (4·38; 2·10 to 6·65) were associated with higher dyspnoea scores. Poor sleep quality, deterioration in sleep quality, and sleep regularity were also associated with impaired lung function, as assessed by forced vital capacity. Depending on the sleep metric, anxiety mediated 18–39% of the effect of sleep disturbance on dyspnoea, while muscle weakness mediated 27–41% of this effect.
Interpretation
Sleep disturbance following hospital admission for COVID-19 is associated with dyspnoea, anxiety, and muscle weakness. Due to the association with multiple symptoms, targeting sleep disturbance might be beneficial in treating the post-COVID-19 condition.
Funding
UK Research and Innovation, National Institute for Health Research, and Engineering and Physical Sciences Research Council.
Introduction
Delayed recovery and persistent illness following hospital admission for COVID-19 have been recognised as constituting post-COVID-19 syndrome.1 Dyspnoea is a frequent symptom of this syndrome, with a recent study suggesting that 48% of patients admitted to hospital with COVID-19 in the UK have dyspnoea.2 Dyspnoea can arise from conditions that affect the respiratory, neurological, cardiovascular, and mental health systems.3 These systems are also affected by sleep disturbance,4 another symptom that has been frequently reported after COVID-19.5, 6, 7, 8, 9, 10, 11, 12, 13, 14 The association between sleep disturbance and dyspnoea, however, has not been widely studied.
Sleep disturbance following hospital admission is common regardless of the original reason for admission.15 Despite its prevalence, the clinical implications of sleep disturbance during recovery from an acute illness are not well understood. In experimental settings, sleep disturbance is causally associated with two recognised causes of dyspnoea: anxiety and muscle weakness.16, 17 Furthermore, epidemiological studies have suggested that sleep disturbance is associated with respiratory disease, which can cause dyspnoea.18 Whether these associations persist following acute sleep disturbance, such as after hospital admission, is yet to be established.
An accurate assessment of sleep disturbance is best carried out with a multimodal approach. Subjective assessments provide an overall score of sleep quality but might be affected by recall (reporting) bias,19 as well as questionnaire language. Subjective assessments also provide only limited insights into specific types of sleep disturbance. By contrast, device-based assessments of sleep quality such as actigraphy20 measure sleep disturbance subtypes but they do not assess overall sleep quality.21 Combining both subjective and device-based measures into a multimodal approach can provide valuable insights into sleep disruption, partially overcoming the limitations of individual approaches.21
Research in context.
Evidence before this study
We systematically searched PubMed for studies (with >100 participants) published between Jan 1, 2020, and Nov 25, 2022, reporting sleep disturbance for patients discharged from hospital after contracting COVID-19, without any language restrictions. Search terms related to COVID-19 (“COVID-19”, “COVID-2019”, “SARS-CoV-2”, “2019-nCoV”, “2019-SARS-CoV-2”), hospitalisation (“hospital*”), sleep (“sleep”), and long-term follow-up (“survivor*”, “recover*”, “persistent”, “follow up”, “long term”, “sequela*”, “long Covid”) were used. We found nine studies reporting that sleep disturbance is a common symptom following hospital admission for COVID-19. The reported prevalence varied between 10% and 70% depending on which subjective method was used. One device-based study suggested that sleep regularity and efficiency are altered but did not report on sleep quality. Most studies only reported the prevalence of sleep disturbance, but two studies also identified an association between sleep disturbance and anxiety. No other clinical associations have been reported, despite COVID-19 symptom studies suggesting that sleep disruption could be part of a cluster of symptoms.
Added value of this study
To the best of our knowledge, this is the largest multicentre cohort study to date investigating the burden and effects of sleep disturbance following hospital admission for COVID-19 in the UK, based on both subjective and device-based metrics. The results of our study confirm that there is a high burden of sleep disturbance after hospital admission for COVID-19. Moreover, sleep disturbance was persistent in our cohort, lasting for at least 1 year after discharge from hospital. Other studies have reported that hospital admission can cause sleep disturbance. Therefore, we compared our findings to all-cause hospital admission in the UK Biobank, revealing a higher burden of sleep disturbance following hospital admission for COVID-19. The clinical associations of sleep disturbance were then assessed, revealing an association with other features of the post-COVID-19 syndrome (ie, dyspnoea and reduced lung function). Subsequent mediation analysis revealed that the association between sleep disturbance and dyspnoea was partially mediated by the effect of sleep disturbance on both anxiety and muscle weakness.
Implications of all the available evidence
Our findings suggest that sleep disturbance is a common problem after hospital admission for COVID-19 and is higher compared to hospital admission for other causes. They also suggest that sleep disturbance is associated with several symptoms, including dyspnoea. Future research should assess whether interventions targeting sleep disturbance can improve dyspnoea by reducing anxiety and improving muscle strength.
Some studies have already reported altered sleep quality following hospital admission for COVID-19.5, 6, 7, 8, 9, 10, 11, 12, 13, 14 The majority of these have been single-centre studies that were modest in size and only used subjective measures. Two studies to date have used a multimodal approach.22, 23 In these studies, an association with anxiety was reported only with subjective but not device-based measures. Furthermore, no other clinical associations were reported. Moreover, the studies only used participants who had been admitted to critical care, thus limiting generalisation to the broader hospital cohort.
We aimed to characterise the prevalence, type, and clinical consequences of sleep disturbance in a broad cohort of patients who had been admitted to hospital for COVID-19 using a multimodal approach. We hypothesised that sleep disturbance would be associated with dyspnoea and that this relationship would be mediated by anxiety and muscle weakness.
Methods
Study design and participants
CircCOVID was a prospective multicentre cohort sub-study designed to investigate the effects of circadian disruption and sleep disturbance on recovery after COVID-19. Participants were recruited from the Post-hospitalisation COVID-19 study (PHOSP-COVID). All participants were aged 18 years or older, admitted to 83 hospitals in the UK with either PCR-confirmed or clinically diagnosed COVID-19, and discharged between March, 2020, and October, 2021. The demographics and recruitment of participants into PHOSP-COVID have been described elsewhere2 and are briefly described in the appendix (p 24). COVID-19 severity during admission was assessed with the WHO clinical progression scale.24 Participants were excluded from the analysis on the basis of pre-existing conditions linked to sleep disturbance, medication, and nosocomial infections (appendix p 24). Written informed consent was obtained from all study participants. The study was approved by the Leeds West Research Ethics Committee (20/YH/0225). PHOSP-COVID is registered on the ISRCTN Registry (ISRCTN10980107).
Procedures
Two different methods were used to subjectively assess sleep quality after discharge from hospital. The first was the Pittsburgh Sleep Quality Index questionnaire.25 The second was the numerical rating scale assessment of sleep quality. Data for both methods were collected at an early timepoint 2–7 months after hospital discharge; for the numerical rating scale, data were also collected at a later timepoint 10–14 months after hospital discharge.
For the device-based assessment of sleep quality, participants were invited to wear a wrist-worn accelerometer (GENEActiv Original, ActivInsights, Kimbolton, UK) on their non-dominant wrist for 24 h per day for 14 days. Details of data cleaning, analysis, and variable definitions are given in the appendix (p 25).
The UK Biobank26 was used as a pre-pandemic comparator cohort for actigraphy data. The UK Biobank recruited 502 540 participants aged 40–69 years who were invited to a baseline visit at one of 22 assessment centres between March, 2006, and July, 2010, during which their phenotypes were established with questionnaires, physical examination, and collection of biological samples. From this dataset, three subcohorts (non-hospitalised individuals, hospitalised individuals, and individuals hospitalised with pneumonia) were created for analysis, and are defined in the appendix (pp 26–28).
Outcomes
The Pittsburgh Sleep Quality Index questionnaire assesses sleep quality across seven components. A total score greater than 5 was defined as poor sleep quality and a score of 5 or less was defined as good sleep quality.25 The numerical rating scale assessment of sleep quality asked patients to rate their sleep quality (0–10, with zero being the worst sleep quality; appendix pp 24–25). Patients who rated their sleep quality as decreasing by one point or more were categorised as reporting deteriorated sleep quality.
The primary outcome for this analysis was breathlessness, assessed using the Dyspnoea-12 validated questionnaire. Other outcomes that were assessed were lung function, anxiety, depression, and muscle function. Details of each assessment are given in the appendix (pp 25–26).
Statistical analysis
Continuous values are presented as means (95% CIs) and ordinal values are presented as medians (IQRs). Unless specified elsewhere, participants with good sleep were compared to participants with poor sleep and participants whose sleep had deteriorated (ie, deterioration in sleep score compared to pre-COVID-19 baseline according to the numerical rating scale) were compared with those whose sleep was unaffected by COVID-19. The top and bottom quintiles were compared for sleep regularity, sleep efficiency, and sleep period duration.27, 28 Sleep regularity was also analysed as a continuous measure; the results are reported in the appendix (p 18). All univariable and multivariable analyses of continuous data were analysed with ordinary least squares linear regression or multinomial logistic regression. The multivariable analyses adjusted for a minimally sufficient set of covariates: age, sex, BMI, number of days into the pandemic, number of days since discharge, pre-COVID-19 comorbidities, COVID-19 severity, and length of stay; participants with missing values for any variable were excluded. This set of covariates was identified on the basis of a directed acyclic graph (appendix pp 28–29). Multinomial logistic regression was used for modelling anxiety. The 95% CIs for regression coefficients were calculated from a residual bootstrap approach with 1999 resamples (appendix p 29). χ2 tests compared the proportions of categorical variables. Dunn's test was use for pairwise comparisons. Mediation was evaluated with linear regression with the product of coefficients method29 to estimate the direct and indirect effects of the relationship, done with the R package lavaan version 0.6-12 (appendix p 30). All data were analysed with R (version 4.2.0) within the Scottish National Safe Haven Trusted Research Environment. A p value less than 0·05 was considered significant. Multiple comparisons were adjusted for using the Bejamini-Hochberg procedure to control the false discovery rate (FDR). Mediation analysis was done as a post-hoc analysis to quantify the strength of the relationships between sleep disturbance, anxiety, muscle weakness, and dyspnoea. We conducted two sensitivity analyses; first, all regressions were repeated with continuous sleep exposures rather than dichotomised (appendix p 18). Second, when matching our cohort to UK Biobank, 25 distinct cohorts were matched and analysed to ensure the results were consistent (appendix p 27).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
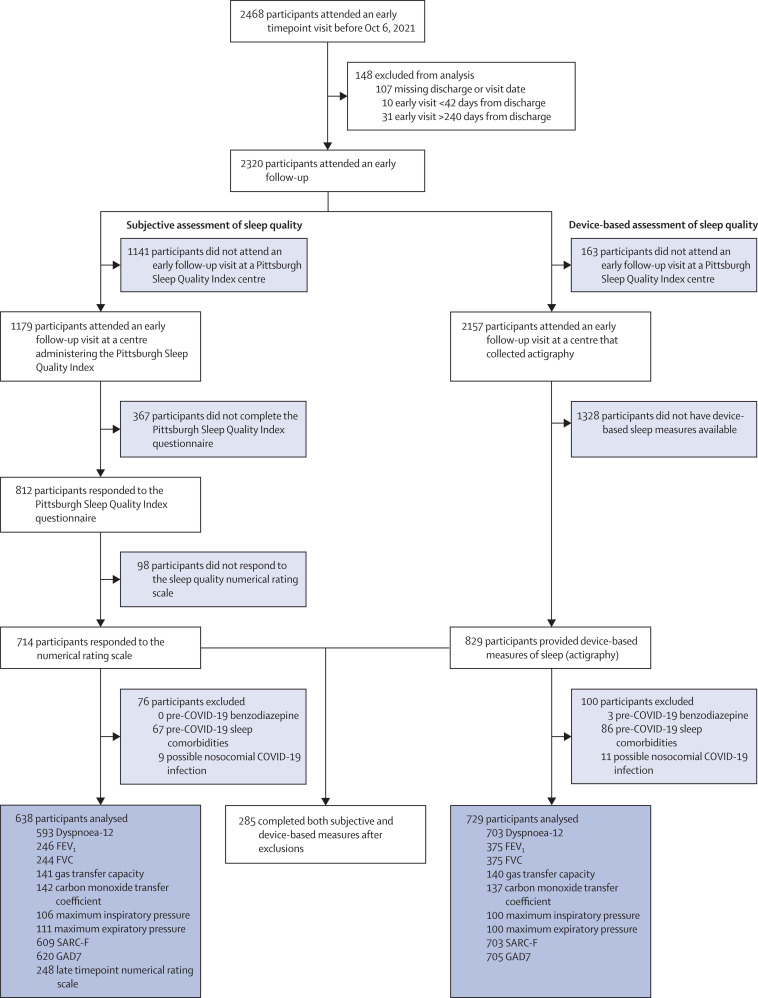
A total of 2468 participants were enrolled in PHOSP-COVID, of whom 2320 attended an early timepoint research visit a median of 5 months (IQR 4–6) following discharge from 83 hospitals in the UK. Subjective sleep quality was measured with both the Pittsburgh Sleep Quality Index questionnaire and the numerical rating scale. At the early timepoint, 1179 (51%) of 2320 participants attended an early follow-up at a centre offering the Pittsburgh Sleep Quality Index questionnaire (figure 1 ). Of these, 714 (61%) of 1179 completed both the Pittsburgh Sleep Quality Index questionnaire and numerical rating scale at the early timepoint. A further 76 (11%) of 714 were excluded owing to suspected nosocomial infection or pre-COVID-19 sleep problems. At the late timepoint, 248 (39%) of 638 participants also completed the numerical rating scale (figure 1), a median of 12 months (IQR 11–13) after discharge. Device-based sleep quality was assessed by actigraphy in 829 (38%) of 2157 eligible participants a median of 7 months (IQR 5–8) after discharge. Nosocomial infection or suspected pre-existing sleep disorder excluded a further 100 (12%) of 829 participants (figure 1). All symptoms were assessed at the first clinical visit a median of 5 months (IQR 4–6) after hospital admission.
Figure 1.
Consort diagram for participants included in the analysis
Participants were recruited from the post-hospitalisation COVID-19 study (PHOSP-COVID) who were evaluated at the early timepoint and gave their consent for participation in the study. Sleep disturbance was evaluated with two types of measures (subjective and device-based). FVC=forced vital capacity. SARC-F=strength, assistance with walking, rising from a chair, climbing stairs, and falls. GAD7=Generalised Anxiety Disorder 7-item scale.
Overall, 285 participants completed both subjective and device-based assessments of sleep quality. Demographics of study participants are presented in the appendix (pp 11–12). When both subjective and device-based groups were compared with each other, and with the broader cohort of participants who consented to participate in this research study, small differences were observed on the basis of when their admission occurred during the pandemic, COVID-19 severity, age, BMI, ethnicity, Townsend deprivation index, days since discharge, and pre-morbid depression and anxiety (appendix pp 11–12).
Participants with poor sleep quality (as defined by the Pittsburgh Sleep Quality Index) tended to be female, younger, have a higher BMI, have previous depression or anxiety, have previous dyspnoea, have previous poorer quality sleep, and have lower alcohol consumption than those with good sleep quality (table ). Similar demographic differences were reported when looking at those who had a deterioration in sleep quality (via the numerical rating scale). However, participants who reported a deterioration in their sleep quality were more likely to have good quality sleep (via the Pittsburgh Sleep Quality Index) before COVID-19 (appendix pp 13–14). Participants with the greatest sleep irregularity following hospital admission for COVID-19 tended to have a lower Townsend deprivation index, be smokers, and have premorbid depression or anxiety, diabetes, hypertension, and kidney disease (appendix pp 15–16).
Table.
Cohort demographics segregated by Pittsburgh Sleep Quality Index
| Good sleep quality (n=242) | Poor sleep quality (n=396) | |||
|---|---|---|---|---|
| Pittsburgh Sleep Quality Index score (n=638) | 3·4 (1·4) | 10·1 (3·4) | ||
| Age, years (n=629) | 59·6 (13·9) | 57·7 (12·4) | ||
| Sex (n=583) | ||||
| Male | 70% (154/221) | 54% (196/362) | ||
| Female | 30% (67/221) | 46% (166/362) | ||
| BMI, kg/m2 (n=565) | 30·6 (6·7) | 32·5 (6·6) | ||
| Ethnicity (n=619) | ||||
| White | 68% (159/234) | 73% (280/385) | ||
| South Asian | 20% (46/234) | 15% (59/385) | ||
| Black | 6% (15/234) | 6% (24/385) | ||
| Mixed race | 3% (7/234) | 2% (9/385) | ||
| Other | 3% (7/234) | 3% (13/385) | ||
| Townsend IMD quintile (n=629) | ||||
| 1 (most deprived) | 18% (44/239) | 21% (83/390) | ||
| 2 | 19% (45/239) | 19% (73/390) | ||
| 3 | 15% (37/239) | 18% (71/390) | ||
| 4 | 22% (52/239) | 22% (84/390) | ||
| 5 (least deprived) | 26% (61/239) | 20% (79/390) | ||
| Smoking status (n=631) | ||||
| Never | 61% (146/239) | 58% (227/392) | ||
| Former smoker | 38% (91/239) | 41% (160/392) | ||
| Current smoker | 1% (2/239) | 1% (5/392) | ||
| Average units of alcohol per week (n=605) | 5·8 (7·5) | 4·3 (7·4) | ||
| Days admission was into pandemic (n=638) | 170 (119) | 176 (118) | ||
| Days since discharge (n=638) | 161 (38) | 162 (41) | ||
| Comorbidities | ||||
| Hypertension (n=576) | 33% (73/221) | 40% (142/355) | ||
| Diabetes (n=571) | 19% (42/220) | 23% (81/351) | ||
| Liver disease (n=571) | 3% (7/220) | 2% (8/351) | ||
| Asthma (n=574) | 14% (31/220) | 16% (57/354) | ||
| COPD (n=573) | 4% (9/220) | 4% (15/353) | ||
| Chronic kidney disease (n=572) | 3% (6/221) | 4% (15/351) | ||
| High cholesterol (n=572) | 24% (54/221) | 22% (78/351) | ||
| Depression or anxiety (n=572) | 5% (12/221) | 15% (51/351) | ||
| COVID-19 severity (n=626) | ||||
| WHO clinical progression | ||||
| WHO class 3–4 | 19% (46/239) | 22% (84/387) | ||
| WHO class 5 | 46% (110/239) | 42% (163/387) | ||
| WHO class 6 | 17% (41/239) | 16% (63/387) | ||
| WHO class 7–9 | 18% (42/239) | 20% (77/387) | ||
| Length of stay, days (n=635) | 13·5 (16·5) | 14·2 (21·0) | ||
| ITU admission (n=631) | 32% (77/241) | 32% (125/390) | ||
| Pre-COVID-19 symptoms (n=638) | ||||
| Subjective sleep quality (10=best) | 9·1 (1·8) | 7·5 (2·7) | ||
| Subjective dyspnoea (0=best) | 0·8 (1·8) | 1·3 (2·1) | ||
| Post-COVID-19 symptoms (n=638) | ||||
| Subjective sleep quality (10=best) | 8·1 (2·5) | 5·2 (2·8) | ||
| Subjective dyspnoea (0=best) | 3·3 (2·8) | 4·5 (2·7) | ||
| PHQ9 level (n=622) | ||||
| None | 80% (189/236) | 36% (140/386) | ||
| Mild | 15% (35/236) | 25% (98/386) | ||
| Moderate | 4% (9/236) | 20% (79/386) | ||
| Moderately severe | <1% (1/236) | 10% (37/386) | ||
| Severe | 1% (2/236) | 8% (32/386) | ||
| GAD7 level (n=620) | ||||
| Minimal | 79% (187/236) | 49% (190/384) | ||
| Mild | 17% (40/236) | 24% (93/384) | ||
| Moderate | 3% (7/236) | 16% (60/384) | ||
| Severe | 1% (2/236) | 11% (41/384) | ||
| Subjective sleep period duration, h (n=603) | 7·4 (1·7) | 6·1 (2·0) | ||
Continuous values are presented as means (SDs) and were compared with a Wilcoxon rank-sum test. Categorical data are presented as percentages (n/N) and were compared with a Pearson χ2 test. A total score greater than 5 on the Pittburgh Sleep Quality Index questionnaire was defined as poor sleep quality and a score of 5 or less was defined as good sleep quality. IMD=Index of Multiple Deprivation. COPD=Chronic obstructive pulmonary disease. ITU=intensive therapy unit. PHQ9=Patient Health Questionnaire. GAD7=Generalised Anxiety Disorder 7-item scale.
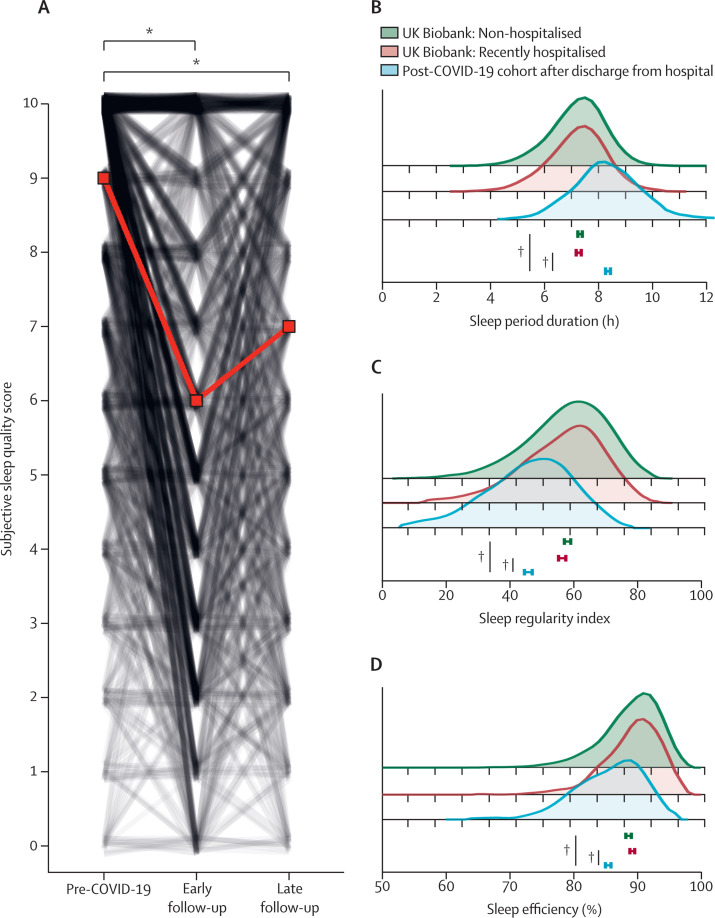
The prevalence of sleep disturbance following COVID-19 hospitalisation was then defined. A subjective assessment of sleep quality revealed that 396 (62%) of 638 participants reported poor sleep quality (assessed by the Pittsburgh Sleep Quality Index). Analysis of temporal changes in sleep quality by the numerical rating scale revealed that sleep quality deteriorated following hospital admission for COVID-19 in 338 (53%) of 638 participants. At the early timepoint, sleep quality fell by a median of 3 (IQR 0–4) points and at the late timepoint sleep quality fell by a median of 2 (0–4) points (figure 2A ) compared to participants' pre-COVID-19 scores.
Figure 2.
Sleep disturbance after hospital admission for COVID-19
(A) Participants were asked to rate their sleep quality before being hospitalised with COVID-19 and at the time of assessment, during their early follow-up (median 5 months after discharge from hospital for COVID-19). Sleep quality was also assessed at a late follow-up (median 12 months after COVID-19 admission). The red line indicates median change; the black lines represent individual participants. *p<0·0001, Dunn's post-hoc test, Benjamini-Hochberg corrected p value. Using a device-based approach, assessments were made for: (B) sleep period duration; (C) sleep regularity index; and (D) sleep efficiency. The post-COVID-19 cohort (blue shaded area) was matched (age, sex, BMI, and, if applicable, time from hospital discharge) to non-hospitalised UK Biobank participants (green shaded area) or recently hospitalised UK Biobank participants (red shaded area). Means are depicted underneath the graphs; the error bars represent 95% CIs. †p<0·0001, t-test Benjamini-Hochberg corrected p value. The p value comparisons are for COVID-19 versus non-hospitalised and COVID-19 versus recently hospitalised cohorts.
The actigraphy traces of this cohort were then compared to two UK Biobank cohorts (non-hospitalised and recently hospitalised), as defined in the appendix (pp 26–27). Cohorts were matched for age, sex, BMI, and, if applicable, time from hospital discharge (appendix p 27). Participants admitted to hospital for COVID-19 slept on average 62 min (95% CI 56 to 68) longer (figure 2B), had a lower sleep regularity index (–21% [95% CI–19 to –23]; figure 2C), and a lower sleep efficiency (3·25 percentage points [95% CI 2·81 to 3·68]; figure 2D) than UK Biobank participants who had not been admitted to hospital (p<0·0001 for all comparisons). Compared to UK Biobank participants who had recently been admitted to hospital, participants admitted to hospital with COVID-19 slept on average 65 min (95% CI 59 to 71) longer (figure 2B), had a lower sleep regularity index (–19% [95% CI –20 to –16]; figure 2C), and a lower sleep efficiency (3·83 percentage points [95% CI 3·40 to 4·26]; figure 2D; p<0·0001 for all comparisons).
Actigraphy traces of 91 participants in the UK Biobank who had recently been admitted to hospital with pneumonia (2–11 months before actigraphy; appendix p 27) were also compared to both UK Biobank cohorts defined above. No significant differences were observed for sleep duration or efficiency compared with either the non-hospitalised or recently hospitalised UK Biobank cohorts (appendix p 20). Participants recently hospitalised with pneumonia did, however, have a lower sleep regularity index (–9% [95% CI –14 to –5]; p=0·0007) than the non-hospitalised UK Biobank cohort (appendix p 20). The small size of this cohort precluded matching to patients admitted to hospital for COVID-19.
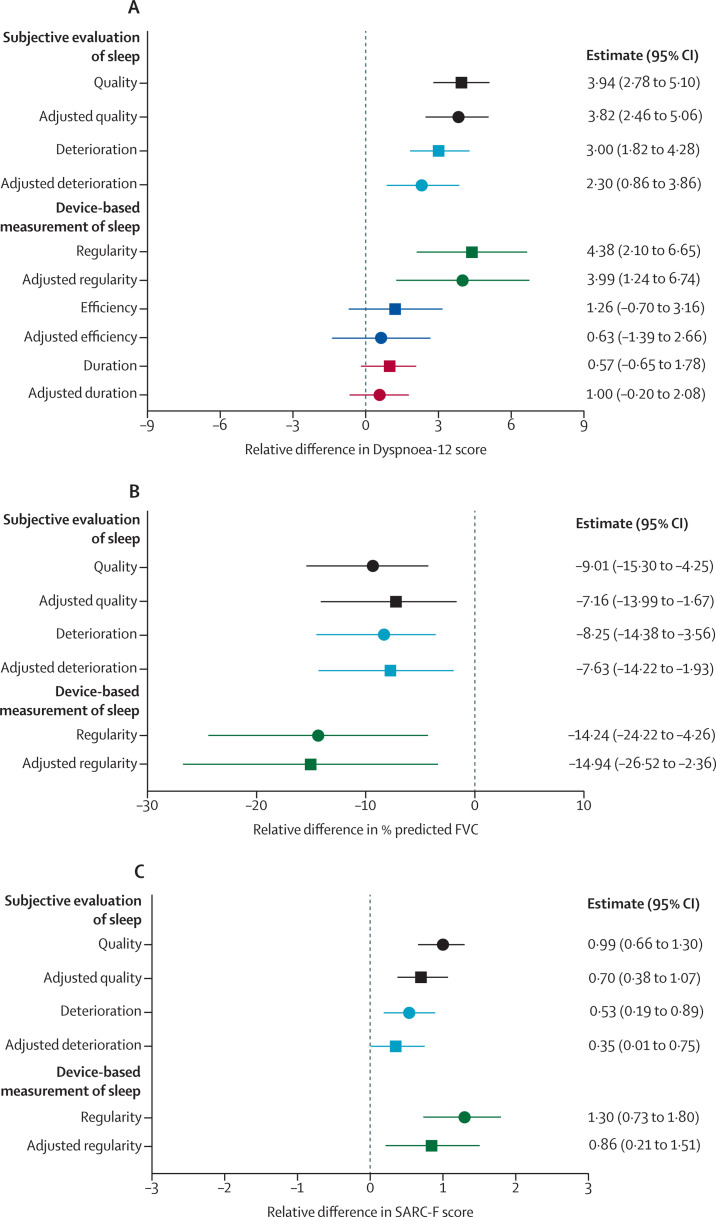
The relationship between sleep disturbance and dyspnoea was then investigated. Participants with poor sleep quality (assessed by the Pittsburgh Sleep Quality Index), scored higher on the Dyspnoea-12 questionnaire than those with good sleep quality (figure 3A ). Sleep quality deterioration (assessed with the numerical rating scale) was also associated with dyspnoea. Those reporting a deterioration in their sleep quality scored higher on the Dyspnoea-12 questionnaire than those who did not have a deterioration in sleep quality (figure 3A). Associations were consistent following adjustments for a minimum set of covariates (age, sex, BMI, period into the pandemic, time since discharge, comorbidities, COVID-19 severity, and length of stay).
Figure 3.
Clinical associations with sleep disturbance
The associations between changes in sleep parameters were investigated for various clinical characteristics: (A) association with Dyspnoea-12 score; (B) association with predicted forced vital capacity; and (C) association with SARC-F score. Sleep quality (ie, poor vs good quality, assessed by the Pittsburgh Sleep Quality Index) is shown in black. Sleep quality deterioration (ie, those whose sleep deteriorated vs those whose sleep was unaffected by COVID-19, assessed by the numerical rating scale) is shown in light blue. Sleep regularity (ie, top vs bottom quintiles) is shown in green. Sleep efficiency (ie, top vs bottom quintiles; dyspnoea only) is shown in dark blue. Sleep period duration (ie, top vs bottom quintiles; dyspnoea only) is shown in red. Both unadjusted (circles) or multivariable (squares) effect estimates are shown alongside 95% CIs. In multivariable linear regression models, the association was adjusted for age, sex, BMI, comorbidities, COVID-19 severity, length of stay, number of days into the pandemic, and number of days since discharge. SARC-F=strength, assistance with walking, rising from a chair, climbing stairs, and falls.
Device-based measurements of sleep were then assessed; participants with the lowest sleep regularity scored higher on the Dyspnoea-12 score than participants with the best sleep regularity (figure 3A; appendix p 18). This association was unaffected following adjustment for a minimum set of covariates. No association was observed between dyspnoea and either sleep efficiency or sleep period duration in both unadjusted and adjusted models (figure 3A). Therefore, these measures were not investigated further.
The relationship between sleep disturbance and lung function was then assessed. Individuals with poor quality sleep (assessed by the Pittsburgh Sleep Quality Index) had a lower predicted FEV1 (–7·09% (95% CI –13·43 to –2·22; appendix pp 18, 21) and a lower predicted forced vital capacity (FVC; figure 3B) than those who reported good quality sleep. The associations were consistent following adjustment for a minimum set of covariates for both FEV1 (appendix pp 18, 21) and FVC (figure 3B; appendix p 18). Participants who had a deterioration in their sleep quality (assessed by the numerical rating scale) following hospital admission for COVID-19 had a lower percentage predicted FEV1 (–8·78%; 95% CI –14·94 to –3·78) and a lower percentage predicted FVC (–8·25%; 95% CI –14·38 to –3·56) than participants whose sleep quality had remained the same or improved. Associations were consistent following adjustments for the minimal set of covariates (figure 3B; appendix pp 18, 21).
Sleep regularity was then assessed. Participants with the lowest sleep regularity had a lower percentage predicted FEV1 (–13·58%; 95% CI –24·86 to –4·84; appendix pp 18, 21) and a lower percentage predicted FVC (figure 3B) than participants with the highest sleep regularity. This association was also consistent following adjustment for a minimal set of covariates (figure 3B; appendix pp 18, 21).
Participants' diffusion capacity was also evaluated. No associations were observed between these measures (carbon monoxide transfer coefficient and diffusing capacity of the lung for carbon monoxide) and the three-sleep metrics for both unadjusted and adjusted models (appendix pp 18, 21).
Participants with the lowest sleep regularity had a lower maximum expiratory pressure (–31·63 cmH2O; 95% CI –58·47 to –3·29; appendix pp 18, 22) than participants with the highest sleep regularity. No similar association was observed with maximum inspiratory pressure. The small sample size (n=55) of this cohort precluded adjustment for a minimal set of covariates. No associations were observed for either maximum inspiratory pressure or maximum expiratory pressure and the subjective measures of sleep quality following hospital admission for COVID-19 (appendix pp 18, 22).
The association between sleep disturbance and muscle function was then assessed. In the assessment of muscle function, participants with poor sleep quality (assessed by the Pittsburgh Sleep Quality Index) had a higher score on the SARC-F questionnaire (figure 3C) than those with good quality sleep. Those who reported sleep quality deterioration (assessed by the numerical rating scale) following hospital admission due to COVID-19 also reported higher scores on the SARC-F questionnaire (figure 3C) than those participants whose sleep had not deteriorated. Associations were consistent following adjustments for a minimal set of covariates (figure 3C; appendix p 18). This association was also observed for sleep irregularity. Participants with the most irregular sleep had a higher SARC-F (figure 3C; appendix p 18) score than participants with the best sleep regularity, with similar results following adjustment.
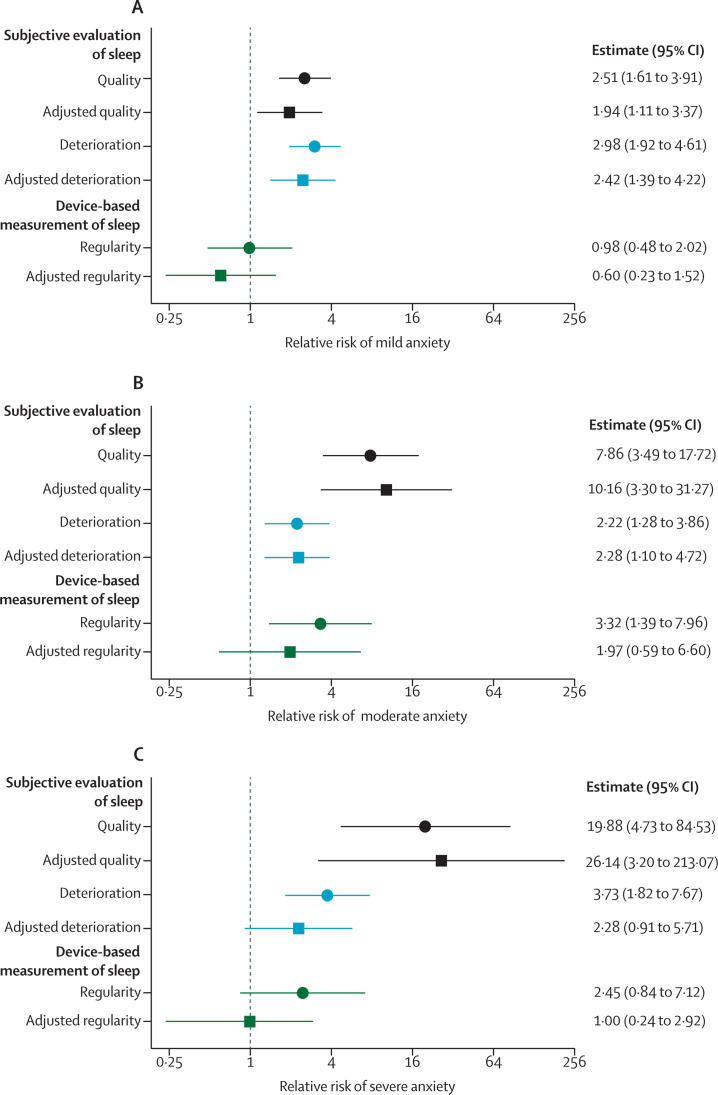
The relationship between sleep disturbance and anxiety was then assessed. Participants with poor sleep quality (assessed by the Pittsburgh Sleep Quality Index) were more likely to have mild, moderate, or severe anxiety compared to participants who reported good quality sleep (figure 4A–C ; appendix p 18).
Figure 4.
Association between sleep disturbance and anxiety
The associations between changes in sleep parameters were investigated with symptoms of anxiety (GAD-7 scale). (A) Relative risk with mild anxiety. (B) Relative risk with moderate anxiety. (C) Relative risk with severe anxiety. Sleep quality (ie, poor vs good quality, assessed by the Pittsburgh Sleep Quality Index) is shown in black. Sleep quality deterioration (ie, those whose sleep deteriorated vs those whose sleep was unaffected by COVID-19, assessed by the numerical rating scale) is shown in light blue. Sleep regularity (ie, top vs bottom quintiles) is shown in green. Both unadjusted (circles) or multivariable (squares) multinomial logistic regression relative risks are shown alongside 95% CIs. In multivariable multinomial logistic regression models, the association was adjusted for age, sex, BMI, comorbidities, COVID-19 severity, length of stay, number of days into the pandemic, and number of days since hospital discharge. The x-axis is on a log2 scale.
A similar association was observed between anxiety and sleep quality deterioration (assessed by the numerical rating scale) after admission to hospital for COVID-19. Participants who had sleep quality deterioration were more likely to have mild, moderate, and severe anxiety (figure 4A–C; appendix p 18) than participants who did not have any deterioration in their sleep quality. Following adjustment for the minimal sufficient set of covariates, the association was attenuated for severe anxiety, but the other associations remained unchanged.
Participants with the lowest sleep regularity were more likely to report moderate anxiety than participants with the highest sleep regularity (figure 4B; appendix p 18). By contrast, there was no association with mild or severe anxiety. Adjustment for the minimal sufficient set of covariates attenuated the effect with moderate anxiety.
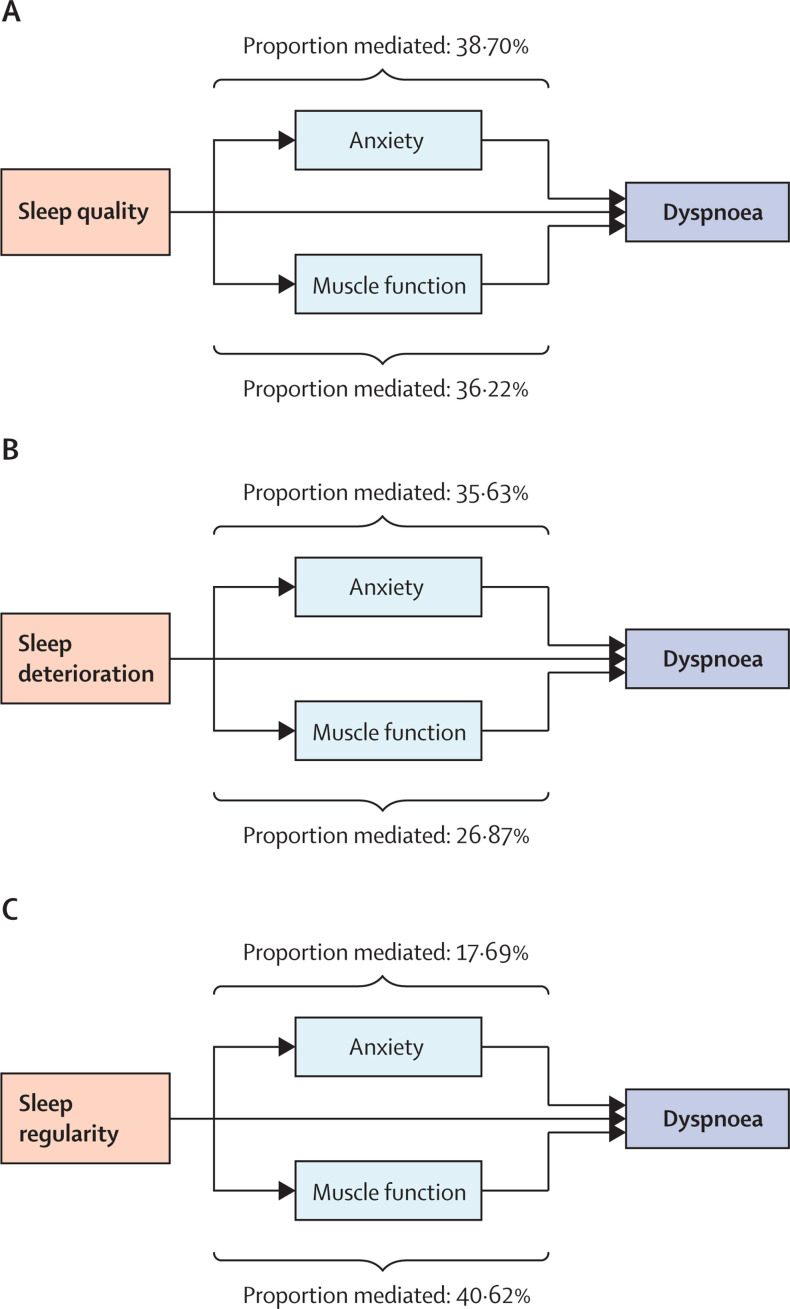
Given that anxiety and altered muscle function are recognised causes of dyspnoea, mediation analysis was done (appendix p 30) to investigate the contribution of anxiety and altered muscle function in mediating the effect between sleep and dyspnoea after admission to hospital due to COVID-19. Anxiety following discharge from hospital mediated the effect of poor sleep quality on dyspnoea by 38·70% (95% CI 22·67–57·17) and reduced muscle function had a similar mediation effect (36·22% [95% CI 21·19–55·66]; figure 5A ; appendix p 19).
Figure 5.
The effect of anxiety or muscle weakness in mediating the effect of sleep on dyspnoea
Mediation models were used to investigate the effects of muscle weakness or anxiety, recognised causes of dyspnoea, in mediating the association between sleep disturbance and dyspnoea.
For the relationship between sleep quality deterioration and dyspnoea, anxiety mediated the effect by 35·63% (95% CI 16·14–59·22) and reduced muscle function mediated the effect by 26·87% (3·91–52·34; figure 5B; appendix p 19). The relationship between sleep irregularity and dyspnoea was also mediated by both anxiety (17·69%; 95% CI 1·38–42·27) and reduced muscle function (40·62%; 15·15–72·34; figure 5C; appendix p 19).
Discussion
Using multimodal sleep evaluation done in a nationwide UK cohort, we have shown that sleep disturbance is prevalent following hospital admission for COVID-19. Moreover, sleep disturbance is likely to persist for at least 12 months, since subjective sleep quality hardly changed between early (5 months) and late (12 months) follow-up visits. Multimodal assessment of sleep disturbance revealed that three factors (sleep quality, degradation of sleep quality compared to baseline, and sleep regularity) were associated with dyspnoea and decreased lung function. Mediation analysis identified that reduced muscle function and anxiety, both recognised causes of dyspnoea,3 could partially mediate the association between sleep disturbance and dyspnoea.
Three different complementary methods (the Pittsburgh Sleep Quality Index, numerical rating scale, and device-based metrics)21 were used to assess sleep disturbance in our study. The Pittsburgh Sleep Quality Index is a well validated assessment tool that evaluates sleep quality at the time of administration.30 Additional evaluation of sleep quality with the numerical rating scale confirmed these associations occurred as a result of a deterioration of sleep quality following hospital admission for COVID-19, complementing the Pittsburgh Sleep Quality Index evaluation. Device-based metrics were then used to investigate specific aspects of sleep quality, revealing clinical associations with sleep irregularity. The gold standard device-based metric is polysomnography. However, this can be technically challenging and measures sleep quality over shorter timeframes. Instead, actigraphy was used, which accurately identifies many of the sleep traits captured by polysomnography.21 Analysis of the actigraphy traces revealed an association between dyspnoea and the sleep regularity index. Although this association has not previously been widely reported, the sleep regularity index has been associated with morbidity in other studies.31, 32, 33
Device-based sleep metrics following hospital admission for COVID-19 have predominantly been measured in participants admitted to critical care.22, 23 Our cohort extends these findings, revealing altered sleep-based metrics in all participants who had been admitted to hospital regardless of critical care admission. Comparison with UK Biobank participants admitted to hospital for other causes suggested this could be partially due to COVID-19, given the comparatively modest effects seen with hospital admission for other causes.
Two previous device-based studies22, 23 in the setting of COVID-19 revealed clinical associations between anxiety and subjective—but not device-based—assessments of sleep quality. These limited clinical effects are an apparent contradiction with experimental models in which sleep disturbance has several effects,34 with clinical studies outside the context of hospital admission,35 and the broad clinical effects reported in the present study. If sleep disturbance does have broad effects in this setting, this could also explain why the association between sleep disturbance and dyspnoea was only partially mediated by anxiety and muscle function. Therefore, other unidentified clinical or behavioural effects or a direct effect could explain the association between sleep disturbance and dyspnoea. Further studies will be needed to define this association further, since the association between sleep disturbance and dyspnoea is likely to be relevant to other respiratory diseases.
Strengths of our study include its size, multicentre design, and the use of different complementary assessment measures to evaluate sleep disturbance. Consistent clinical associations were also observed across each evaluation method. However, this study also has some limitations that should be considered when interpreting the results. First, the hypothesised directionality of effects in the directed acyclic graph (appendix pp 28–29, 32), used to identify the covariates in our models, cannot be confirmed by the data in this study. Other studies do support the hypothesised directions;36, 37 however, bidirectionality relationships have been reported in other settings.16 Second, quantification of sleep quality deterioration based on the numerical rating scale relied upon participant recall and therefore could be affected by recall bias, also known as reporting bias.19 Last, selection bias could also affect the results. However, we minimised this bias by using bootstrapping combined with cohort matching.
In conclusion, this study provides insight into the prevalence and wider consequences of sleep disturbance following hospital admission for COVID-19. The associations described in this study between sleep disturbance and reduced muscle function, anxiety, and dyspnoea suggest that sleep disturbance could be an important driver of the post-COVID-19 condition. If this is the case, then interventions targeting poor sleep quality38 could be used to manage multimorbidity and convalescence following hospital admission for COVID-19, with the aim of potentially improving patient outcomes.
Data sharing
The PHOSP-COVID study protocol, consent form, definition, and derivation of clinical characteristics and outcomes, training materials, regulatory documents, information about requests for data access, and other relevant study materials are available online. UK Biobank information can be released once necessary approvals have been obtained. Other data (eg, the R code and protocol) will be made available on reasonable request to the corresponding author.
Declaration of interests
IDS declares a statistical editor honoraria role with Thorax. TP declares support from the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre (BRC) to complete the work. ALH declares an editor-in-chief role for Computer Physics Communications. RA declares speaker fees and travel support from Boehringer Ingelheim. CEB declares their institute was awarded a grant from the UK Research and Innovation (UKRI)/NIHR and institutional support from NIHR Nottingham BRC to complete this work; the author reports grants from Nottingham Hospitals Charity and Nottingham University Hospitals Research and Innovation Department. TC declares support from the NIHR South London and Maudsley NHS Foundation Trust, King's College London BRC to complete the work; the author reports grants from Guy's and St Thomas' Charity, NIHR, and UKRI; the author has published self-help books on chronic fatigue for which she receives royalties; the author has received ad-hoc payments for workshops carried out in long-term conditions; the author declares travel and accommodation support; the author is part of the scientific committee of the British Association for Behavioural and Cognitive Psychotherapies (BABCP) and she is on the expert advisory panel for COVID-19 Rapid Guidelines. JDC declares grants from AstraZeneca, Novartis, Boehringer Ingelheim, Genentech, Gilead Sciences, Insmed, GlaxoSmithKline, and Grifols; the author reports consulting fees from AstraZeneca, Insmed, Boehringer Ingelheim, Janssen, Antabio, Chiesi, Novartis, Pfizer, Zambon, GlaxoSmithKline, and Grifols. GD declares support from the NIHR Imperial BRC, Royal Brompton and Harefield NGS Foundation Trust, Royal Brompton & Harefield Hospitals Charity, Imperial College Healthcare NHS Trust, and the Medical Research Council (MRC) to complete this work; the author reports grants from Genentech, AstraZeneca, British Lung Foundation (BLF) Early Cohort, GlaxoSmithKline, Novartis, Chiesi, and Boehringer Ingelheim; the author has a published chapter in a textbook for which he receives payment; the author declares a role in the advisory boards for AstraZeneca and Novartis and as honorarium as deputy editor of the American Journal of Respiratory and Critical Care Medicine (AJRCCM). L-PH declares grants from NIHR Oxford BRC. MGJ declares grants from the Royal Society, MRC, BLF, Boehringer Ingelheim, and the Asthma, Allergy and Inflammatory Research Charity. BR declares support from the British Heart Foundation (BHF) Oxford Centre of Research Excellence (CRE) to complete this work. SLR-J declares support for her institute from UKRI to complete the work; the author reports grants from UKRI, The European and Developing Countries Clinical Trials Partnership (EDCTP), MRC, Rosetrees Trust, and the Global Challenges Research Fund (GCRF); the author reports an honorarium for chapter contribution from the Federation of European Academies of Medicine; the author is Data and Safety Monitoring Board (DSMB) Chair for a Bexsero trial funded by Wellcome Trust; the author declares an editor role for AIDS journal. AVR declares support from NIHR Leicester BRC to complete the work. MS declares support for his institute from MRC/Department of Health and Social Care (DHSC) to complete the work. ASi declares support for her institute from UKRI/NIHR to complete the work. DGW declares an advanced Fellowship grant from NIHR; the author receives an honorarium from bioMérieux to present. TY declares support from the NIHR Leicester BRC to complete the work. RGJ declares grants from AstraZeneca, Biogen, Galecto, GlaxoSmithKline, Nordic Biosciences, RedX, Pliant; the author received consulting fees from AstraZeneca, Brainomix, Bristol Myers Squibb, Chiesi, Cohbar, Daewoong, GlaxoSmithKline, Veracyte, Resolution Therapeutics, and Pliant; the author has received payments from Boehringer Ingelheim, Chiesi, Roche, patientMpower, and AstraZeneca; the author was paid for expert testimony by Pinsent Masons LLP; the author has participated on a DSMB for Boehringer Ingelheim, Galapagos, and Vicore; the author has held a leadership role at NuMedii and is President of Action for Pulmonary Fibrosis. SJS declares grants from NIHR, Wellcome Doctoral Training Programme (DTP), the Human Tissue Authority, NIHR DHSC/UKRI COVID-19 Rapid Response Initiative, NIHR Global Research Group, Actegy Limited, and as an NIHR Senior Investigator; the author has presented for GlaxoSmithKline, Ministry of Justice, CIPLA, and Sherbourne Gibbs; the author is on the National Institute for Health and Care Excellence (NICE) Expert Advisor Panel for Long COVID and was on the Wales Long COVID Advisory Board; the author is the American Thoracic Society (ATS) Pulmonary Rehabilitation Assembly Chair, Clinical Lead Royal Society of Physicians (RSP) Pulmonary Rehabilitation Accreditation Scheme, and Clinical Lead Nation Asthma and COPD Audit Programme (NACAP) for Pulmonary Rehabilitation. WD-CM declares grants for his institution from NIHR, National Health Service (NHS) Accelerated Access Collaborative, and BLF; the author is the honorary President of the Association for Respiratory Technology and Physiology but receives no payment. CEBr declares support from UKRI/DHSC and NIHR Leicester BRC to complete the work; the author reports grants from GlaxoSmithKline, AstraZeneca, Sanofi, CI, Chiesi, Novartis, Roche, Genentech, Mologic, and 4DPharma; the author received consulting fees paid to his institution from GlaxoSmithKline, AstraZeneca, Sanofi, BI, Chiesi, Novartis, Roche, Genentech, Mologic, 4DPharma, and TEVA. LVW declares support from UKRI, GlaxoSmithKline/Asthma + Lung UK, and NIHR to complete this work; the author receives grants from Orion Pharma, GlaxoSmithKline, Genentech, and AstraZeneca; the author received consulting fees paid to her institution from Galapagos and Boehringer Ingelheim; the author received support for attending a meeting from Genentech. JCP received consulting fees from Istesso and The Limbic; the author participated on a DSMB for Vicore. AART declares a fellowship grant from BHF and a grant from NIHR to his institution; the author received an honorarium for lectures from Janssen-Cilag Ltd; the author received support for attending meetings from Janssen-Cilag Ltd. AH declares support from UKRI, NIHR, and NIHR Manchester BRC to complete the work; the author is Chair NIHR Translational Research Collaboration (unpaid). PLM declares a grant for his institution from AstraZeneca; the author received consulting fees from Hoffmann-La Roche, Boehringer Ingelheim, AstraZeneca, Trevi, and Qureight; the author received speaker fees from Boehringer Ingelheim and Hoffmann-La Roche. RAE declares support from UKRI/MRC to complete the work; the author reports a grant from NIHR/Wolfson Foundation; the author received consulting fees from AstraZeneca for Long COVID; the author received a speaker fee from Boehringer Ingelheim for a lecture on Long COVID; the author received support to attend BTS conference virtually from Chiesi; the author is the European Respiratory Society Group 01.02 Pulmonary Rehabilitation Secretary (unpaid). JFB declares support to his institute from an MRC Transition Fellowship, Asthma + Lung UK, NIHR Manchester BRC, and UKRI; the author reports grants paid to his institution from the Small Business Research Initiative Home Spirometer and the National Institute of Academic Anaesthesia; the author has received support for attending meetings from TEVA and Therakos; the author is a committee member of the Royal Society of Medicine (RSM). All other authors declare no competing interests.
Acknowledgments
Acknowledgments
CJ is funded by an Engineering and Physical Sciences Research Council (EPSRC) Mathematics Doctoral Training Partnership (EP/W523884/1). L-PH is supported in part by the Oxford NIHR Biomedical Research Centre. PLM is supported by an Action for Pulmonary Fibrosis Mike Bray Fellowship and an Asthma + Lung UK Chair in Respiratory Research. KP-H receives funding from Innovate UK (TS/T013028/1) and the UK Medical Research Council (MRC; MR/W006111/1). JCP receives funding from the NIHR University College London Hospitals Biomedical research centre and Breathing Matters charity. BR is funded by the British Heart Foundation Oxford Centre of Research Excellence (RE/18/3/34214). ABD is funded by a Wellcome fellowship (216606/Z/19/Z). AART is supported by a British Heart Foundation intermediate clinical fellowship (FS/18/13/33281). LVW is supported by the GlaxoSmithKline/Asthma + Lung UK Chair in Respiratory Research (C17-1). DGW is funded by an NIHR Advanced Fellowship (NIHR300669). RGJ is supported by a NIHR Research Professorship (RP-2017-08-ST2-014). JFB and PSC are supported by an MRC transition support fellowship (MR/T032529/1). IDS is supported by a fellowship funded by The Rayne Foundation. The work was also supported by an Asthma + Lung UK Malcolm Walleans Grant. This work was supported by the NIHR Manchester Biomedical Research Centre (grant number NIHR203308; JFB, MKR, and AH). The study was also funded by UK Research and Innovation and National Institute of Health Research (grant references: EP/V051490/1, MR/V027859/1, MR/W006111/1, and COV0319). PHOSP-COVID is jointly funded by a grant from the MRC-UK Research and Innovation and the Department of Health and Social Care through the National Institute for Health Research (NIHR) rapid response panel to tackle COVID-19 grant references above. The views expressed in the publication are those of the authors and not necessarily those of the National Health Service (NHS), the NIHR, or the Department of Health and Social Care. This study would not be possible without all the participants who gave their time and support. We thank all the participants and their families. We thank the many research administrators, health-care and social-care professionals who contributed to setting up and delivering the study at all the 65 NHS trusts and health boards and 25 research institutions across the UK, as well as all the supporting staff at the NIHR Clinical Research Network, the Health Research Authority, the Research Ethics Committee, Department of Health and Social Care, Public Health Scotland, and Public Health England, and support from the ISARIC Coronavirus Clinical Characterisation Consortium. This research was done with the UK Biobank Resource under application number 6818. We thank the participants and researchers from the UK Biobank who contributed or collected data. We thank Kate Holmes at the NIHR Office for Clinical Research Infrastructure (NOCRI) for her support in coordinating the charities group. The PHOSP-COVID industry framework was formed to provide advice and support in commercial discussions, and we thank the Association of the British Pharmaceutical Industry as well the NIHR Office for Clinical Research Infrastructure (NOCRI) for coordinating this work. We are grateful to all the charities that provided insights for this study: Action Pulmonary Fibrosis, Alzheimer's Research UK, Asthma + Lung UK, British Heart Foundation, Diabetes UK, Cystic Fibrosis Trust, Kidney Research UK, MQ Mental Health, Muscular Dystrophy UK, Stroke Association Blood Cancer UK, McPin Foundations, and Versus Arthritis. We thank the NIHR Leicester Biomedical Research Centre patient and public involvement group and Long Covid Support. We also thank the reviewers whose thoughtful comments improved the quality of the Article.
Contributors
The manuscript was initially drafted by CJ, IDS, MKR, and JFB, and further developed by the writing committee. CJ, IDS, NC, MKR, and JFB made substantial contributions to the conception and design of this work. CJ, IDS, JCP, AART, ALH, PLM, RAE, and TP made substantial contributions to the acquisition of data. CJ, IDS, TP, PSC, ALH, BA-S, RA, CEB, TC, JDC, NC, ABD, GD, CLE, OE, NJG, NAH, VCH, EMH, L-PH, LH-W, LSH, CJJ, MGJ, OCL, KEL, NIL, MM, HJCMc, MAMc, BVP, KP-H, KP, BR, MR, PR-O, SLR-J, AVR, RMS, JTS, MS, AMS, AS, ASi, SCS, MT, DGW, TY, RGJ, SJS, WD-CM, CEBr, LVW, JCP, AART, AH, PLM, RAE, SEJ, MKR, and JFB made contributions to the analysis or interpretation of data for this work. CJ, IDS, RAE, and TP verified the underlying data. All authors contributed to data interpretation and critical review and revision of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Contributor Information
PHOSP-COVID Study Collaborative Group:
C Jackson, I D Stewart, T Plekhanova, P S Cunningham, A L Hazel, B Al-Sheklly, R Aul, C E Bolton, T Chalder, J D Chalmers, N Chaudhuri, A B Docherty, G Donaldson, C L Edwardson, O Elneima, N J Greening, N A Hanley, V C Harris, E M Harrison, L-P Ho, L Houchen-Wolloff, L S Howard, C J Jolley, M G Jones, O C Leavy, K E Lewis, N I Lone, M Marks, H J C McAuley, M A McNarry, B Patel, K Piper-Hanley, K Poinasamy, B Raman, M Richardson, P Rivera-Ortega, S L Rowland-Jones, A V Rowlands, R M Saunders, J T Scott, M Sereno, A M Shah, A Shikotra, A Singapuri, S C Stanel, M Thorpe, D G Wootton, T Yates, G Jenkins, S J Singh, W D-C Man, C E Brightling, L V Wain, J C Porter, R Thompson, A Horsley, P L Molyneaux, R A Evans, S E Jones, M K Rutter, J F Blaikley, K Abel, H Adamali, D Adeloye, O Adeyemi, R Adrego, L A Aguilar Jimenez, S Ahmad, N Ahmad Haider, R Ahmed, N Ahwireng, M Ainsworth, B Al-Sheklly, A Alamoudi, M Ali, M Aljaroof, AM All, L Allan, R J Allen, L Allerton, L Allsop, P Almeida, D Altmann, M Alvarez Corral, S Amoils, D Anderson, C Antoniades, G Arbane, A Arias, C Armour, L Armstrong, N Armstrong, D Arnold, H Arnold, A Ashish, A Ashworth, M Ashworth, S Aslani, H Assefa-Kebede, C Atkin, P Atkin, H Aung, L Austin, C Avram, A Ayoub, M Babores, R Baggott, J Bagshaw, D Baguley, L Bailey, J K Baillie, S Bain, M Bakali, M Bakau, E Baldry, D Baldwin, M Baldwin, C Ballard, A Banerjee, B Bang, R E Barker, L Barman, S Barratt, F Barrett, D Basire, N Basu, M Bates, A Bates, R Batterham, H Baxendale, H Bayes, M Beadsworth, P Beckett, M Beggs, M Begum, P Beirne, D Bell, R Bell, K Bennett, E Beranova, A Bermperi, A Berridge, C Berry, S Betts, E Bevan, K Bhui, M Bingham, K Birchall, L Bishop, K Bisnauthsing, J Blaikely, A Bloss, A Bolger, J Bonnington, A Botkai, C Bourne, M Bourne, K Bramham, L Brear, G Breen, J Breeze, A Briggs, E Bright, S Brill, K Brindle, L Broad, A Broadley, C Brookes, M Broome, A Brown, J Brown, J S Brown, M Brown, V Brown, T Brugha, N Brunskill, M Buch, P Buckley, A Bularga, E Bullmore, L Burden, T Burdett, D Burn, G Burns, A Burns, J Busby, R Butcher, A Butt, S Byrne, P Cairns, P C Calder, E Calvelo, H Carborn, B Card, C Carr, L Carr, G Carson, P Carter, A Casey, M Cassar, J Cavanagh, M Chablani, R C Chambers, F Chan, K M Channon, K Chapman, A Charalambou, A Checkley, J Chen, Y Cheng, L Chetham, C Childs, E R Chilvers, H Chinoy, A Chiribiri, K Chong-James, G Choudhury, N Choudhury, P Chowienczyk, C Christie, M Chrystal, D Clark, C Clark, J Clarke, S Clohisey, G Coakley, Z Coburn, S Coetzee, J Cole, C Coleman, F Conneh, D Connell, B Connolly, L Connor, A Cook, B Cooper, J Cooper, S Cooper, D Copeland, T Cosier, M Coulding, C Coupland, E Cox, T Craig, P Crisp, D Cristiano, M G Crooks, A Cross, I Cruz, P Cullinan, D Cuthbertson, L Daines, M Dalton, P Daly, A Daniels, P Dark, J Dasgin, A David, C David, E Davies, F Davies, G Davies, G A Davies, K Davies, M J Davies, J Dawson, E Daynes, A De Soyza, B Deakin, A Deans, C Deas, J Deery, S Defres, A Dell, K Dempsey, E Denneny, J Dennis, A Dewar, R Dharmagunawardena, N Diar-Bakerly, C Dickens, A Dipper, S Diver, S N Diwanji, M Dixon, R Djukanovic, H Dobson, S L Dobson, A Donaldson, T Dong, N Dormand, A Dougherty, R Dowling, S Drain, K Draxlbauer, K Drury, P Dulawan, A Dunleavy, S Dunn, C Dupont, J Earley, N Easom, C Echevarria, S Edwards, C Edwardson, H El-Taweel, A Elliott, K Elliott, Y Ellis, A Elmer, D Evans, H Evans, J Evans, R Evans, R I Evans, T Evans, C Evenden, L Evison, L Fabbri, S Fairbairn, A Fairman, K Fallon, D Faluyi, C Favager, T Fayzan, J Featherstone, T Felton, J Finch, S Finney, J Finnigan, L Finnigan, H Fisher, S Fletcher, R Flockton, M Flynn, H Foot, D Foote, A Ford, D Forton, E Fraile, C Francis, R Francis, S Francis, A Frankel, E Fraser, R Free, N French, X Fu, J Fuld, J Furniss, L Garner, N Gautam, J R Geddes, J George, P M George, M Gibbons, M Gill, L Gilmour, F Gleeson, J Glossop, S Glover, N Goodman, C Goodwin, B Gooptu, H Gordon, T Gorsuch, M Greatorex, P L Greenhaff, W Greenhalf, A Greenhalgh, J Greenwood, H Gregory, R Gregory, D Grieve, D Griffin, L Griffiths, A-M Guerdette, B Guillen Guio, M Gummadi, A Gupta, S Gurram, E Guthrie, Z Guy, H Henson, K Hadley, A Haggar, K Hainey, B Hairsine, P Haldar, I Hall, L Hall, M Halling-Brown, R Hamil, A Hancock, K Hancock, S Haq, H E Hardwick, E Hardy, T Hardy, B Hargadon, K Harrington, E Harris, P Harrison, N Hart, A Harvey, M Harvey, M Harvie, L Haslam, M Havinden-Williams, J Hawkes, N Hawkings, J Haworth, A Hayday, M Haynes, J Hazeldine, T Hazelton, L G Heaney, C Heeley, J L Heeney, M Heightman, S Heller, M Henderson, L Hesselden, M Hewitt, V Highett, T Hillman, T Hiwot, L-P Ho, A Hoare, M Hoare, J Hockridge, P Hogarth, A Holbourn, S Holden, L Holdsworth, D Holgate, M Holland, L Holloway, K Holmes, M Holmes, B Holroyd-Hind, L Holt, A Hormis, A Hosseini, M Hotopf, K Howard, A Howell, E Hufton, A D Hughes, J Hughes, R Hughes, A Humphries, N Huneke, E Hurditch, J Hurst, M Husain, T Hussell, J Hutchinson, W Ibrahim, F Ilyas, J Ingham, L Ingram, D Ionita, K Isaacs, K Ismail, T Jackson, J Jacob, W Y James, W Jang, C Jarman, I Jarrold, H Jarvis, R Jastrub, B Jayaraman, R G Jenkins, P Jezzard, K Jiwa, C Johnson, S Johnson, D Johnston, D Jones, G Jones, H Jones, I Jones, L Jones, M G Jones, S Jones, S Jose, T Kabir, G Kaltsakas, V Kamwa, N Kanellakis, S Kaprowska, Z Kausar, N Keenan, S Kelly, G Kemp, S Kerr, H Kerslake, A L Key, F Khan, K Khunti, S Kilroy, B King, C King, L Kingham, J Kirk, P Kitterick, P Klenerman, L Knibbs, S Knight, A Knighton, O Kon, S Kon, S S Kon, S Koprowska, A Korszun, I Koychev, C Kurasz, P Kurupati, C Laing, H Lamlum, G Landers, C Langenberg, D Lasserson, L Lavelle-Langham, A Lawrie, C Lawson, A Layton, A Lea, D Lee, J-H Lee, E Lee, K Leitch, R Lenagh, D Lewis, J Lewis, V Lewis, N Lewis-Burke, X Li, T Light, L Lightstone, W Lilaonitkul, L Lim, S Linford, A Lingford-Hughes, M Lipman, K Liyanage, A Lloyd, S Logan, D Lomas, R Loosley, J M Lord, H Lota, W Lovegrove, A Lucey, E Lukaschuk, A Lye, C Lynch, S MacDonald, G MacGowan, I Macharia, J Mackie, L Macliver, S Madathil, G Madzamba, N Magee, M M Magtoto, N Mairs, N Majeed, E Major, F Malein, M Malim, G Mallison, S Mandal, K Mangion, C Manisty, R Manley, K March, S Marciniak, P Marino, M Mariveles, E Marouzet, S Marsh, B Marshall, M Marshall, J Martin, A Martineau, L M Martinez, N Maskell, D Matila, W Matimba-Mupaya, L Matthews, A Mbuyisa, S McAdoo, H McAllister-Williams, A McArdle, P McArdle, D McAulay, G P McCann, J McCormick, W McCormick, P McCourt, L McGarvey, C McGhee, K Mcgee, J McGinness, K McGlynn, A McGovern, H McGuinness, I B McInnes, J McIntosh, E McIvor, K McIvor, L McLeavey, A McMahon, M J McMahon, L McMorrow, T Mcnally, M McNarry, J McNeill, A McQueen, H McShane, C Mears, C Megson, S Megson, P Mehta, J Meiring, L Melling, M Mencias, D Menzies, M Merida Morillas, A Michael, C Miller, L Milligan, C Mills, G Mills, N L Mills, L Milner, S Misra, J Mitchell, A Mohamed, N Mohamed, S Mohammed, W Monteiro, S Moriera, A Morley, L Morrison, R Morriss, A Morrow, A J Moss, P Moss, K Motohashi, N Msimanga, E Mukaetova-Ladinska, U Munawar, J Murira, U Nanda, H Nassa, M Nasseri, A Neal, R Needham, P Neill, S Neubauer, D E Newby, H Newell, T Newman, J Newman, A Newton-Cox, T Nicholson, D Nicoll, A Nikolaidis, C M Nolan, M J Noonan, C Norman, P Novotny, J Nunag, L Nwafor, U Nwanguma, J Nyaboko, C O'Brien, K O'Donnell, D O'Regan, L O'Brien, N Odell, G Ogg, O Olaosebikan, C Oliver, Z Omar, P J M Openshaw, L Orriss-Dib, L Osborne, R Osbourne, M Ostermann, C Overton, J Owen, J Oxton, J Pack, E Pacpaco, S Paddick, S Painter, A Pakzad, S Palmer, P Papineni, K Paques, K Paradowski, M Pareek, D Parekh, H Parfrey, C Pariante, S Parker, M Parkes, J Parmar, S Patale, M Patel, S Patel, D Pattenadk, M Pavlides, S Payne, L Pearce, J E Pearl, D Peckham, J Pendlebury, Y Peng, C Pennington, I Peralta, E Perkins, Z Peterkin, T Peto, N Petousi, J Petrie, P Pfeffer, J Phipps, J Pimm, R Pius, H Plant, S Plein, M Plowright, O Polgar, L Poll, J Porter, S Portukhay, N Powell, A Prabhu, J Pratt, A Price, C Price, D Price, L Price, A Prickett, J Propescu, S Prosper, S Pugmire, S Quaid, J Quigley, J Quint, H Qureshi, I N Qureshi, K Radhakrishnan, N M Rahman, M Ralser, A Ramos, H Ramos, J Rangeley, B Rangelov, L Ratcliffe, P Ravencroft, A Reddington, R Reddy, A Reddy, H Redfearn, D Redwood, A Reed, M Rees, T Rees, K Regan, W Reynolds, C Ribeiro, A Richards, E Richardson, K Roberts, E Robertson, E Robinson, L Robinson, L Roche, C Roddis, J Rodger, A Ross, G Ross, J Rossdale, A Rostron, A Rowe, A Rowland, J Rowland, M J Rowland, S L Rowland-Jones, K Roy, M Roy, I Rudan, R Russell, E Russell, G Saalmink, R Sabit, E K Sage, T Samakomva, N Samani, C Sampson, K Samuel, R Samuel, A Sanderson, E Sapey, D Saralaya, J Sargent, C Sarginson, T Sass, N Sattar, K Saunders, P Saunders, L C Saunders, H Savill, W Saxon, A Sayer, J Schronce, W Schwaeble, K Scott, N Selby, M G Semple, T A Sewell, K Shah, P Shah, M Shankar-Hari, M Sharma, C Sharpe, M Sharpe, S Shashaa, A Shaw, K Shaw, V Shaw, A Sheikh, S Shelton, L Shenton, K Shevket, J Short, S Siddique, S Siddiqui, J Sidebottom, L Sigfrid, G Simons, J Simpson, N Simpson, C Singh, S J Singh, D Sissons, J Skeemer, K Slack, A Smith, D Smith, S Smith, J Smith, L Smith, M Soares, T S Solano, R Solly, A R Solstice, T Soulsby, D Southern, D Sowter, M Spears, L G Spencer, F Speranza, L Stadon, S Stanel, N Steele, M Steiner, D Stensel, G Stephens, L Stephenson, M Stern, R Stimpson, S Stockdale, J Stockley, W Stoker, R Stone, W Storrar, A Storrie, K Storton, E Stringer, S Strong-Sheldrake, N Stroud, C Subbe, C L Sudlow, Z Suleiman, C Summers, C Summersgill, D Sutherland, D L Sykes, R Sykes, N Talbot, A L Tan, L Tarusan, V Tavoukjian, A Taylor, C Taylor, J Taylor, A Te, H Tedd, C J Tee, J Teixeira, H Tench, S Terry, S Thackray-Nocera, F Thaivalappil, B Thamu, D Thickett, C Thomas, D C Thomas, S Thomas, A K Thomas, T Thomas-Woods, T Thompson, A A R Thompson, T Thornton, R S Thwaites, J Tilley, N Tinker, G F Tiongson, M Tobin, J Tomlinson, C Tong, M Toshner, R Touyz, K A Tripp, E Tunnicliffe, A Turnbull, E Turner, S Turner, V Turner, K Turner, S Turney, L Turtle, H Turton, J Ugoji, R Ugwuoke, R Upthegrove, J Valabhji, M Ventura, J Vere, C Vickers, B Vinson, E Wade, P Wade, T Wainwright, L O Wajero, S Walder, S Walker, E Wall, T Wallis, S Walmsley, J A Walsh, S Walsh, L Warburton, T J C Ward, K Warwick, H Wassall, S Waterson, E Watson, L Watson, J Watson, J Weir McCall, C Welch, H Welch, B Welsh, S Wessely, S West, H Weston, H Wheeler, S White, V Whitehead, J Whitney, S Whittaker, B Whittam, V Whitworth, A Wight, J M Wild, M Wilkins, D Wilkinson, B Williams, N Williams, J Williams, S A Williams-Howard, M Willicombe, G Willis, J Willoughby, A Wilson, D Wilson, I Wilson, N Window, M Witham, R Wolf-Roberts, C Wood, F Woodhead, J Woods, J Wormleighton, J Worsley, D Wraith, C Wrey Brown, C Wright, L Wright, S Wright, J Wyles, I Wynter, M Xu, N Yasmin, S Yasmin, K P Yip, B Young, S Young, A Young, A J Yousuf, A Zawia, L Zeidan, B Zhao, B Zheng, and O Zongo
Supplementary Material
References
- 1.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans RA, McAuley H, Harrison EM, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9:1275–1287. doi: 10.1016/S2213-2600(21)00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374 doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 4.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843–844. doi: 10.5665/sleep.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacho-Hernández JC, Fernández-de-Las-Peñas C, Fuensalida-Novo S, Jiménez-Antona C, Ortega-Santiago R, Cigarán-Mendez M. Sleep quality mediates the effect of sensitization-associated symptoms, anxiety, and depression on quality of life in individuals with post-COVID-19 pain. Brain Sci. 2022;12 doi: 10.3390/brainsci12101363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolattürk ÖF, Soylu AC. Evaluation of cognitive, mental, and sleep patterns of post-acute COVID-19 patients and their correlation with thorax CT. Acta Neurol Belg. 2022;2022:1–5. doi: 10.1007/s13760-022-02001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández-de-Las-Peñas C, Martín-Guerrero JD, Florencio LL, et al. Clustering analysis reveals different profiles associating long-term post-COVID symptoms, COVID-19 symptoms at hospital admission and previous medical co-morbidities in previously hospitalized COVID-19 survivors. Infection. 2022;51:61–69. doi: 10.1007/s15010-022-01822-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frontera JA, Yang D, Medicherla C, et al. Trajectories of neurologic recovery 12 months after hospitalization for COVID-19: a prospective longitudinal study. Neurology. 2022;99:e33–e45. doi: 10.1212/WNL.0000000000200356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magnúsdóttir I, Lovik A, Unnarsdóttir AB, et al. Acute COVID-19 severity and mental health morbidity trajectories in patient populations of six nations: an observational study. Lancet Public Health. 2022;7:e406–e416. doi: 10.1016/S2468-2667(22)00042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-de-Las-Peñas C, Martín-Guerrero JD, Cancela-Cilleruelo I, Moro-López-Menchero P, Rodríguez-Jiménez J, Pellicer-Valero OJ. Trajectory curves of post-COVID anxiety/depressive symptoms and sleep quality in previously hospitalized COVID-19 survivors: the LONG-COVID-EXP-CM multicenter study. Psychol Med. 2022 doi: 10.1017/S003329172200006X. published online Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu L, Fang Y, Luo D, et al. Pre-hospital, in-hospital and post-hospital factors associated with sleep quality among COVID-19 survivors 6 months after hospital discharge: cross-sectional survey in five cities in China. BJPsych Open. 2021;7:e191. doi: 10.1192/bjo.2021.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Islam MK, Molla MMA, Hasan P, et al. Persistence of sleep disturbance among post-COVID patients: findings from a 2-month follow-up study in a Bangladeshi cohort. J Med Virol. 2022;94:971–978. doi: 10.1002/jmv.27397. [DOI] [PubMed] [Google Scholar]
- 13.Fernández-de-Las-Peñas C, Gómez-Mayordomo V, de-la-Llave-Rincón AI, et al. Anxiety, depression and poor sleep quality as long-term post-COVID sequelae in previously hospitalized patients: a multicenter study. J Infect. 2021;83:496–522. doi: 10.1016/j.jinf.2021.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Li T, Chen L, et al. Association of sleep quality before and after SARS-CoV-2 infection with clinical outcomes in hospitalized patients with COVID-19 in China. EXCLI J. 2021;20:894–906. doi: 10.17179/excli2021-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altman MT, Knauert MP, Pisani MA. Sleep disturbance after hospitalization and critical illness: a systematic review. Ann Am Thorac Soc. 2017;14:1457–1468. doi: 10.1513/AnnalsATS.201702-148SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chellappa SL, Aeschbach D. Sleep and anxiety: From mechanisms to interventions. Sleep Med Rev. 2022;61 doi: 10.1016/j.smrv.2021.101583. [DOI] [PubMed] [Google Scholar]
- 17.Thun E, Bjorvatn B, Flo E, Harris A, Pallesen S. Sleep, circadian rhythms, and athletic performance. Sleep Med Rev. 2015;23:1–9. doi: 10.1016/j.smrv.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Kim JS, Dashti HS, Huang T, et al. Associations of sleep duration and sleep-wake rhythm with lung parenchymal abnormalities on computed tomography: the MESA study. J Sleep Res. 2022;31 doi: 10.1111/jsr.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baillet M, Cosin C, Schweitzer P, et al. Mood influences the concordance of subjective and objective measures of sleep duration in older adults. Front Aging Neurosci. 2016;8:181. doi: 10.3389/fnagi.2016.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takemura N, Cheung DST, Fong DYT, et al. Relationship of subjective and objective sleep measures with physical performance in advanced-stage lung cancer patients. Sci Rep. 2021;11 doi: 10.1038/s41598-021-96481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Langenberg SCN, Kocevska D, Luik AI. The multidimensionality of sleep in population-based samples: a narrative review. J Sleep Res. 2022;31 doi: 10.1111/jsr.13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Targa ADS, Benítez ID, González J, et al. Sleep and circadian health 6 months after critical COVID-19 disease. Respirology. 2022;27:1083–1088. doi: 10.1111/resp.14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benítez ID, Moncusí-Moix A, Vaca R, et al. Sleep and circadian health of critical COVID-19 survivors 3 months after hospital discharge. Crit Care Med. 2022;50:945–954. doi: 10.1097/CCM.0000000000005476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 26.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lunsford-Avery JR, Engelhard MM, Navar AM, Kollins SH. Validation of the sleep regularity index in older adults and associations with cardiometabolic risk. Scientific reports. 2018;8(1):1–11. doi: 10.1038/s41598-018-32402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips AJK, Clerx WM, O'Brien CS, et al. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci Rep. 2017;7 doi: 10.1038/s41598-017-03171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacKinnon DP. Routledge; New York, NY: 2012. Introduction to statistical mediation analysis. [Google Scholar]
- 30.Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73. doi: 10.1016/j.smrv.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Omichi C, Koyama T, Kadotani H, et al. Irregular sleep and all-cause mortality: a large prospective cohort study. Sleep Health. 2022;8:678–683. doi: 10.1016/j.sleh.2022.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Culver MN, McMillan NK, Cross BL, et al. Sleep duration irregularity is associated with elevated blood pressure in young adults. Chronobiol Int. 2022;39:1320–1328. doi: 10.1080/07420528.2022.2101373. [DOI] [PubMed] [Google Scholar]
- 33.Huang T, Mariani S, Redline S. Sleep irregularity and risk of cardiovascular events: the multi-ethnic study of atherosclerosis. J Am Coll Cardiol. 2020;75:991–999. doi: 10.1016/j.jacc.2019.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin M, Chen Y, Zheng H, et al. Assessment of mouse cognitive and anxiety-like behaviors and hippocampal inflammation following a repeated and intermittent paradoxical sleep deprivation procedure. Behav Brain Res. 2017;321:69–78. doi: 10.1016/j.bbr.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 35.Ramar K, Malhotra RK, Carden KA, et al. Sleep is essential to health: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2021;17:2115–2119. doi: 10.5664/jcsm.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pellitteri G, Surcinelli A, De Martino M, et al. Sleep alterations following COVID-19 are associated with both neuroinflammation and psychological disorders, although at different times. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.929480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bethea TN, Zhai W, Zhou X, et al. Associations between longitudinal changes in sleep disturbance and depressive and anxiety symptoms during the COVID-19 virus pandemic among older women with and without breast cancer in the thinking and living with breast cancer study. Cancer Med. 2022;11:3352–3363. doi: 10.1002/cam4.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.García-Serrano C, Pujol Salud J, Aran-Solé L, et al. Enhancing night and day circadian contrast through sleep education in prediabetes and type 2 diabetes mellitus: a randomized controlled trial. Biology. 2022;11:893. doi: 10.3390/biology11060893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The PHOSP-COVID study protocol, consent form, definition, and derivation of clinical characteristics and outcomes, training materials, regulatory documents, information about requests for data access, and other relevant study materials are available online. UK Biobank information can be released once necessary approvals have been obtained. Other data (eg, the R code and protocol) will be made available on reasonable request to the corresponding author.