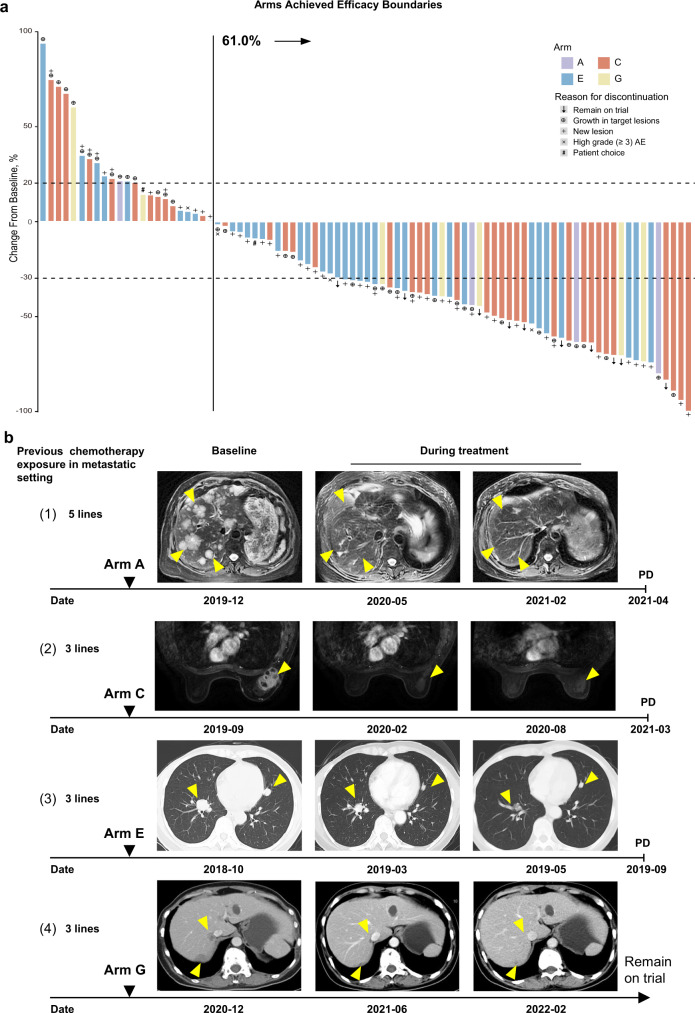
Fig. 3. Tumor responses in arms that achieved prespecified efficacy boundary.
a Best percent change in the sum of target lesion diameters (longest diameter for nonnodular lesions and short axis for nodal lesions) from baseline for 105 patients in arms A, C, E, G. The dashed lines at +20% and −30% indicate thresholds for progressive disease and partial response, respectively, according to RECIST v1.1. Eighty-seven patients in the 4 arms with postbaseline tumor assessments of target lesions are represented in the plot, while 18 patients without postbaseline tumor assessments of target lesions are not shown. b Examples of patients with objective responses. Yellow arrows indicate metastatic lesions. (1) A 66-year-old woman with mTNBC that progressed after 5 lines of previous treatment in the metastatic setting was identified as having the LAR subtype with an ERBB2 D769Y mutation; this patient was enrolled in arm A (December 2019). She received pyrotinib and capecitabine therapy and achieved a PR 1.6 months after the initiation of therapy. In April 2021, her intrahepatic lesions progressed. Baseline images of intrahepatic diffuse metastases and typical images of tumor regression during treatment are shown. (2) A 70-year-old woman with mTNBC that progressed after 3 lines of previous treatment in the metastatic setting was identified as having the IM subtype; this patient was enrolled in arm C (September 2019). She received anti-PD-1 therapy combined with nab-paclitaxel and achieved a PR 2.0 months after the initiation of therapy. She discontinued nab-paclitaxel and immunotherapy due to AEs in February and September 2020, respectively. She was followed up closely until March 2021, when her breast lesion had progressed. Images of the target lesion (right breast) before and during treatment are shown. (3) A 53-year-old female with mTNBC that progressed after 3 lines of previous therapy in the metastatic setting was identified as having the BLIS subtype; this patient was enrolled in arm E (October 2018). She received apatinib monotherapy and achieved a PR 1.9 months after the initiation of therapy. In September 2019, her lung metastases progressed. Computed tomography scans before and during treatment are shown. (4) A 54-year-old woman with mTNBC that progressed after 3 lines of previous treatment in the metastatic setting was identified as having the MES subtype with a PIK3CA H1047R mutation; this patient was enrolled in arm G (January 2021). She received everolimus with nab-paclitaxel therapy and achieved a PR 2.0 months after the initiation of therapy. She discontinued nab-paclitaxel chemotherapy after 10 cycles of therapy due to grade II neurotoxicity. In close follow-up, the patient’s disease remained stable for 14 months until March 2022. Images of the patient’s liver metastases before and during treatment are shown. AE adverse event, PD progressive disease, mTNBC metastatic triple-negative breast cancer, LAR luminal androgen receptor, IM immunomodulatory, BLIS basal-like immune-suppressed, MES mesenchymal-like.