Fig. 8. Molecular model of methyltransferase NozMT docked with tolerated substrate cyclo-D-Trp-C3’-prenyl-D-Trp (6) and rejected substrate DD-cWW (5).
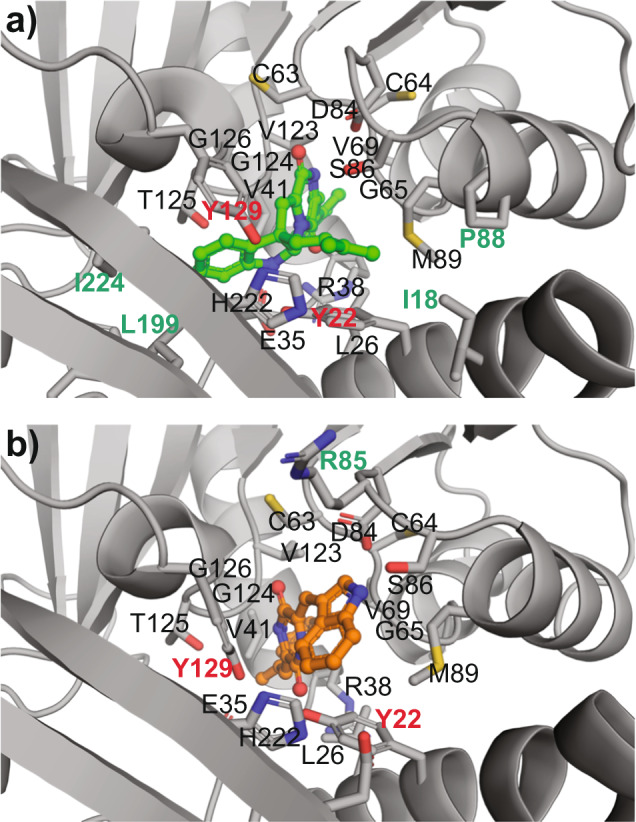
a Docking of 6 (green carbon atoms) with NozMT gave a binding ΔG of −12.6 kcal/mol. b Docking of 5 (orange carbon atoms) gave an inferior ΔG of −10.4 kcal/mol. In (a)–(b), NozMT residues within 4 Å of each candidate substrate are rendered as licorice sticks and labeled. Residues unique to NozMT binding of 5 vs. 6 are labeled in green, and suggest additional hydrophobic interactions with 6 (via Ile-18, Pro-88, Leu-199, Ile-224). Candidate catalytic residues Tyr-22 and Tyr-129, targeted in site-directed mutagenesis experiments, are labeled in bold red. Supplementary Data 2–3 provide PDB files of these docking structures.
