Abstract
Background
Magnetic sphincter augmentation (MSA) erosion, disruption or displacement clearly requires device removal. However, up to 5.5% of patients without anatomical failure require removal for dysphagia or recurrent GERD symptoms. Studies characterizing these patients or their management are limited. We aimed to characterize these patients, compare their outcomes, and determine the necessity for further reflux surgery.
Methods
This is a retrospective review of 777 patients who underwent MSA at our institution between 2013 and 2021. Patients who underwent device removal for persistent dysphagia or recurrent GERD symptoms were included. Demographic, clinical, objective testing, and quality of life data obtained preoperatively, after implantation and following removal were compared between removal for dysphagia and GERD groups. Sub-analyses were performed comparing outcomes with and without an anti-reflux surgery (ARS) at the time of removal.
Results
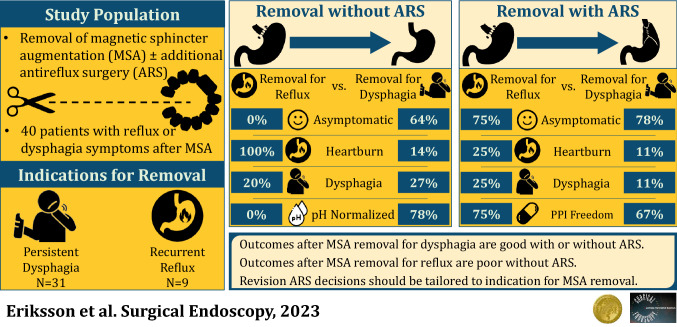
A total of 40 (5.1%) patients underwent device removal, 31 (77.5%) for dysphagia and 9 (22.5%) for GERD. After implantation, dysphagia patients had less heartburn (12.9-vs-77.7%, p = 0.0005) less regurgitation (16.1-vs-55.5%, p = 0.0286), and more pH-normalization (91.7-vs-33.3%, p = 0.0158). Removal without ARS was performed in 5 (55.6%) GERD and 22 (71.0%) dysphagia patients. Removal for dysphagia patients had more complete symptom resolution (63.6-vs-0.0%, p = 0.0159), freedom from PPIs (81.8-vs-0.0%, p = 0.0016) and pH-normalization (77.8-vs-0.0%, p = 0.0455).
Patients who underwent removal for dysphagia had comparable symptom resolution (p = 0.6770, freedom from PPI (p = 0.3841) and pH-normalization (p = 0.2534) with or without ARS. Those who refused ARS with removal for GERD had more heartburn (100.0%-vs-25.0%, p = 0.0476), regurgitation (80.0%-vs-0.0%, p = 0.0476) and PPI use (75.0%-vs-0.0%, p = 0.0476).
Conclusions
MSA removal outcomes are dependent on the indication for removal. Removal for dysphagia yields excellent outcomes regardless of anti-reflux surgery. Patients with persistent GERD had worse outcomes on all measures without ARS. We propose a tailored approach to MSA removal-based indication for removal.
Graphical abstract
Keywords: Magnetic sphincter augmentation, Device removal, Gastroesophageal reflux disease, Symptom recurrence, Dysphagia
Magnetic sphincter augmentation (MSA) is a highly effective anti-reflux surgery (ARS) in patients with gastroesophageal reflux disease (GERD) [1]. Studies have shown significant improvement in GERD-health-related quality of life (HRQL) score and high rates of freedom from antisecretory medication and pH normalization following this procedure. [2–4] The advantage of the MSA, compared to Nissen fundoplication, is that it is reversible, technically standardized, largely preserves gastric anatomy and has lower rates of side effects such as gas-bloat syndrome [5]. However, despite its excellent efficacy and superior side effect profile, dysphagia remains the most common complaint after MSA [6]. Up to 31% of patients with persistent post-operative dysphagia require at least one endoscopic dilation [7]. Although, dysphagia can be managed effectively with diet and dilation in the majority of patients, some will require device removal [7]. Published removal rates range from 0.5 to 8.3% of patients, with persistent dysphagia or recurrent GERD symptoms often listed as the most common indications for removal. [8–12] The management for anatomic failures such as recurrent hernia, erosion, device displacement or device disruption is straightforward: surgical revision. However, the approach to the symptomatic patient with anatomic integrity is less clear. This population and their device removal management regimens have not been well described in the literature.
The current study was designed to characterize the groups of patients who required device removal for recurrent GERD symptoms or persistent dysphagia despite no objective evidence of device erosion, displacement or disruption. The clinical outcomes after device removal, and the necessity for further treatment of post-removal GERD symptoms were assessed. Based on these results, we proposed a treatment paradigm to assist with management of patients who are being considered for device removal.
Material and methods
Study design and population
This was a retrospective review of prospectively collected data from patients who underwent magnetic sphincter augmentation (MSA) between 2013 and 2021 at the Esophageal Institute, Allegheny Health Network (AHN) (Pittsburgh, PA). Inclusion criteria were patients with an age of 18 years or older with no history of foregut surgery prior to MSA, who underwent device removal for either persistent dysphagia or recurrent GERD symptoms and had at least 6 months post-removal follow-up. This study was reviewed and approved by the Institutional Review Board of the AHN (IRB 2021–259).
Evaluation prior to MSA
All patients underwent complete foregut evaluation prior to index surgery, consisting of a detailed clinical examination, esophagogastroduodenoscopy (EGD), video-esophagram and esophageal physiology testing such as pH monitoring and manometry. Additionally, patients were asked to complete standardized questionnaires including the GERD health-related quality of life (GERD-HRQL) survey [13]. The GERD-HRQL objectively assesses overall patient satisfaction and severity of reflux symptoms using a 0 to 5 rating scale. Esophageal motility was assessed by high-resolution manometry (HRM). Once off proton pump inhibitors for 10 days, patients underwent ambulatory wireless 48-h Bravo® pH monitoring (Medtronics, Shoreview, MN). [14] A DeMeester score > 14.7 was considered abnormal acid exposure to the distal esophagus.
Surgical technique for MSA
The LINX® reflux management system (Ethicon, Johnson & Johnson; Shoreview, MN), consists of interlinked magnetic titanium beads and features a Roman Arch design assuring non-compressing device closure [15]. Its dynamic design ensures that the esophageal range of motion is not limited.
All procedures were performed laparoscopically by experienced foregut surgeons using standardized surgical techniques as previously described. The procedure consists of complete mediastinal dissection to obtain adequate intraabdominal esophageal length (≥ 2 cm), posterior crural closure and device placement at the level of the gastroesophageal junction with the posterior vagus nerve trunk located on the outside of the device. This approach was used in all patients regardless of whether there was a hiatal hernia. Many patients have transverse widening of the hiatal opening with minimal axial displacement and our approach is focused on restoring the crural contribution of the antireflux barrier during device placement. Intraoperative EGD was performed in order to assist in identifying the anatomic GEJ and to assess device position.
A solid “LINX” diet was started on the day of surgery to avoid development of dysphagia due to formation of scar tissue surrounding the device. Patients were encouraged to eat small portions of solid food every hour while awake for the first two postoperative months. Discharge from hospital occurred on the day of surgery in majority of patients.
Post device implantation assessment
Postoperative follow-up visits were routinely scheduled at 2 weeks, 6 weeks, 6 months and annually after MSA. Patients were assessed for clinical resolution of their symptoms and freedom from anti-secretory medications. At our institution, the typical post-operative assessment regimen after MSA included 6 month and annual GERD-HRQL assessments, and at one-year postoperative patients were approached to repeat their esophageal physiology testing. Patients who reported dysphagia within the first 8 weeks after surgery were initially managed with dietary modification and observed, as the majority of early post-operative dysphagia resolves without intervention. If patients continued to complain of persistent dysphagia as defined by a score > 3 on the GERD-HRQL “difficulty swallowing” item 8 weeks after surgery, despite following dietary recommendations, they were considered for fluoroscopically guided sequential through-the-scope balloon dilation of the magnetically augmented lower esophageal sphincter. Patients who underwent dilation received a seven-day course of twice daily 20 mg prednisone. Patients who failed to respond to three dilations or had recurrent reflux symptoms were considered for device removal.
Surgical technique of device removal
Device removal was performed laparoscopically by the implanting surgeon in most cases. The outer fibrous capsule surrounding the LINX device was opened with Harmonic Shears (Ethicon, Johnson and Johnson, Raritan, NJ) or laparoscopic scissors and each anterior bead was sequentially freed of its capsular attachments. Once sufficient exposure was achieved, the device was disconnected either by disarticulating the clasp or cutting the interconnecting wire with Harmonic shears. Further capsular dissection followed, systematically freeing the posterior beads while applying gentle traction until the device was free. Whenever possible, the device was removed in one piece through the abdominal port. The inner capsule, in contact with the esophagus and anterior vagus nerve, and the small portion of outer capsule in the region of the posterior vagus nerve were left unviolated. After removal from the body, the beads were counted and the surgical sites and surrounding tissues were carefully inspected for injury. Upper endoscopy was performed to confirm no perforation or bleeding. Hiatal hernia repair and/or further anti-reflux procedures were then performed, if indicated.
Post LINX® removal assessment and outcomes
After device removal, patients were assessed using a similar post-operative clinic schedule as post-implantation patients. Patients were divided into two groups based on whether persistent post-operative dysphagia (removal for dysphagia group) or persistent or recurrent reflux symptoms (removal for GERD group) was the indication for device removal. Baseline demographics and objective testing results were compared between two groups. Additionally, primary GERD symptoms (heartburn, regurgitation, dysphagia), PPI use, and objective esophageal physiology testing at baseline, post-implantation, and post-removal were compared for each group.
The possibility of performing an additional ARS was discussed with all patients prior to device removal. All patients with objective evidence of recurrent GERD were recommended ARS at the time of removal. The final decision as to whether or not to proceed with an additional ARS was determined by shared decision making, factoring in surgeon recommendation and patient preference. Sub-analyses comparing those who underwent additional ARS at the time of device removal to those who refused ARS were performed.
Statistical analysis
Data are described as median (interquartile range), mean (standard deviation), or frequency (percent), where appropriate. Statistical analysis was performed using non-parametric tests. Categorical variables were assessed using the Fisher exact test and continuous data using the Wilcoxon Rank test or Kruskal–Wallis test, as appropriate. A p value < 0.05 was considered to be statistically significant. All statistical analyses were performed using SAS software (version 9.4, SAS Institute).
Results
A total of 777 patients underwent MSA between 2013 and 2021 at our institution. Of these, 40 (5.1%) patients had device removal for recurrent GERD or persistent dysphagia. There were 9 (22%) patients in removal for GERD group and 31 (77%) in removal for dysphagia group. Baseline demographic and clinical characteristics are summarized in Table 1. The total population was 71% female with a median (IQR) BMI of 29.1 (22.5–31.9). Demographics and baseline clinical characteristics were comparable between groups. Pre-operative objective testing results are summarized in Table 2. Patients in the removal for GERD group had significantly more LA grade C or D esophagitis than patients in the removal for dysphagia group (22.2 vs. 0.0%, p = 0.0462). Patients who underwent removal for dysphagia were more frequently implanted with a smaller sized device (13 or 14 beads) than the removal for GERD group, but this difference was not significant (87.1 vs. 55.6%, p = 0.0594).
Table 1.
Baseline demographic and clinical characteristics
| Total population (n = 40) |
Recurrent GERD (n = 9) |
Dysphagia (n = 31) |
p-value | |
|---|---|---|---|---|
|
Age, median (IQR), years |
52.0 (37.0–62.5) |
37.0 (31.0–56.0) |
53 (45.0–63.0) |
0.1084 |
| Sex | ||||
| Male, n (%) | 9 (29.0%) | 4 (44.4%) | 5 (16.1%) | 0.1680 |
| Female, n (%) | 31 (71.0%) | 5 (55.6%) | 26 (83.9%) | |
|
BMI, median (IQR), kg/m2 |
29.1 (22.5–31.9) |
30.3 (24.0–31.3) |
27.7 (20.9–32.0) |
0.7095 |
| BMI ≥ 30, n (%) | 19 (47.5%) | 5 (55.6%) | 14 (45.2%) | 0.7116 |
| GERD Symptoms | ||||
| Typical, n (%) | 24 (60.0%) | 7 (77.8%) | 17 (54.8%) | 0.2717 |
| Atypical, n (%) | 16 (40.0%) | 2 (22.2%) | 14 (45.2%) | |
|
GERD-HRQL, Median (IQR), Total Score |
39.0 (17.0–52.0) |
46.0 (23.0–53.5) |
39.0 (14.5–49.5) |
0.7164 |
| Dysphagia, n (%) | 7 (17.5%) | 0 (0.0%) | 7 (22.6%) | 0.1747 |
Table 2.
Pre-operative objective testing
| Recurrent GERD (n = 9) | Dysphagia (n = 31) | p-value | |
|---|---|---|---|
| Hiatal hernia, n (%) | 6 (66.7%) | 28 (90.3%) | 0.1147 |
| Hiatal hernia > 3 cm, n (%) | 0 (0.0%) | 1 (3.2%) | 1.0000 |
| Esophagitis, n (%) | 5 (55.6%) | 9 (29.0%) | 0.2338 |
| LA C + D, n (%) | 2 (22.2%) | 0 (0.0%) | 0.0462 |
| History of Barrett’s, n (%) | 1 (11.1%) | 3 (9.7%) | 1.0000 |
| DeMeester Score, Median (IQR) | 44.4 (18.7–68.0) | 20.0 (15.3–29.8) | 0.1770 |
| DeMeester Score > 14.7, n (%) | 7 (77.8%) | 23 (74.2%) | 1.0000 |
| Manometric features, median (IQR) | |||
| LES length | 2.8 (2.2–3.1) | 3.2 (2.6–4.0) | 0.1355 |
| Intrabdominal length | 1.1 (0.0–1.4) | 1.5 (0.1–2.5) | 0.2184 |
| Resting pressure | 25.6 (12.6–28.9) | 29.7 (21.5–33.4) | 0.1521 |
| Residual pressure | 10.0 (6.7–10.3) | 9.7 (7.1–11.0) | 0.8759 |
| Distal contractile integral | 1764 (999.4–3166) | 2019 (1518–3073) | 0.6288 |
| Distal wave amplitude | 95.2 (57.5–123.8) | 93.3 (78.8–119.3) | 0.8342 |
| Percent peristalsis | 95.0 (85.0–100.0) | 100.0 (90.0–100.0) | 0.6520 |
| Incomplete bolus clearance | 5.0 (0.0–70.0) | 0.0 (0.0–30.0) | 0.3712 |
Post-implantation outcomes
Subjective and objective surgical outcomes after MSA are summarized in Table 3. Among the patients in the removal for dysphagia group, 29% reported either heartburn or regurgitation. However, they had significantly lower rates of heartburn (12.9 vs. 77.7%, p = 0.0005) and regurgitation (16.1 vs. 55.5%, p = 0.0286) compared to patients in the removal for GERD group. Dysphagia was present in 44.4% of removal for GERD patients and 100.0% of removal for dysphagia patients (p = 0.0002). There was a significant reduction in the median GERD-HRQL total scores after device implantation in the removal for dysphagia group (39.0 to 17.5, p = 0.0465), but not in the removal for GERD group (p = 0.2934). Normalization of distal esophageal pH was achieved by 91.7% in the removal for dysphagia group as opposed to only 33.3% in the removal for GERD group (p = 0.0158). Manometry revealed that patients in the removal for dysphagia group had higher median residual pressures (19.2 vs. 14.3, p = 0.156) and rates of incomplete bolus clearance (40.0 vs. 0.0%, p = 0.0452) compared to patients in the removal for GERD group.
Table 3.
Post-implant clinical and objective findings
| Recurrent GERD (n = 9) | Dysphagia (n = 31) | p-value | |
|---|---|---|---|
| GERD symptoms | |||
| Heartburn, n (%) | 7 (77.8%) | 4 (12.9%) | 0.0005 |
| Regurgitation, n (%) | 5 (55.6%) | 5 (16.1%) | 0.0286 |
| Dysphagia, n (%) | 4 (44.4%) | 31 (100%) | 0.0002 |
| GERD-HRQL score, median (IQR) | 18 (6–43.8) | 17.5 (13–27.5) | 0.7615 |
| Freedom from PPI, n (%) | 6 (75.0%) | 17 (85.0%) | 0.6056 |
| Satisfaction, n (%) | 3 (37.5%) | 8 (40.0%) | 1.0000 |
| DeMeester score, median (IQR)* | 20.8 (9.2–42.6) | 6.5 (1.7–11.7) | 0.0329 |
| pH Normalization, n (%)* | 3 (33.3%) | 11 (91.7%) | 0.0158 |
| Manometric features, median (IQR)† | |||
| LES Length | 2.9 (2.5–3.4) | 3.3 (2.5–3.8) | 0.4185 |
| Intra-abdominal length | 1.3 (0.6–1.9) | 2.3 (1.5–2.9) | 0.1476 |
| Resting pressure | 25.9 (23.8–31.8) | 31.1 (25.1–36.7) | 0.2664 |
| Residual pressure | 14.3 (11.0–18.7) | 19.2 (17.5–23.2) | 0.0156 |
| Distal contractile integral | 1888 (1148–3294) | 2144 (1461–4422) | 0.6027 |
| Distal wave amplitude | 88.4 (70.8–125.5) | 78.0 (47.2–98.1) | 0.4665 |
| Percent peristalsis | 100.0 (100.0–100.0) | 95.0 (50.0–100.0) | 0.0612 |
| Incomplete bolus clearance | 0.0 (0.0–10.0) | 40.0 (0.0–60.0) | 0.0452 |
*12 Dysphagia group patients underwent post-implant Bravo
†21 Dysphagia group patients underwent post-implant manometry
Removal outcomes
Device removal was performed at a median (IQR) of 23.7 (13.0–27.2) months in the removal for GERD group and 10.6 (5.4–16.7) months in the removal for dysphagia group (p = 0.0951). Post-removal follow-up assessments were performed at a median (IQR) of 11.7 (5.0–22.8) months.
There were 5 (55.6%) patients in the removal for GERD group and 22 (71.0%) patients in the removal for dysphagia group that underwent device removal without additional ARS. Clinical and objective outcomes following device removal for these patients are summarized in Table 4. Complete resolution of the typical GERD symptoms was achieved by 63.6% of removal for dysphagia and 0.0% of removal for GERD patients (p = 0.0159). Patients who underwent removal for GERD had significantly higher rates of heartburn (100.0 vs. 13.6%, p = 0.0007) and regurgitation (80.0 vs 13.6%, p = 0.0089). Rates of post-removal dysphagia were comparable between groups (p = 1.0000). The removal for dysphagia group was significantly more likely to achieve freedom from anti-secretory medications (81.8 vs. 0.0%, p = 0.0016). In a subset of 12 patients (3 removal for GERD; 9 removal for dysphagia) who underwent post-removal pH-monitoring, patients who underwent removal for dysphagia were significantly more likely to achieve pH normalization (77.8 vs. 0.0%, p = 0.0455).
Table 4.
Post-removal without ARS clinical and objective findings
| Recurrent GERD (n = 5) | Dysphagia (n = 22) | p-value | |
|---|---|---|---|
| GERD symptoms | |||
| Complete resolution, n (%) | 0 (0.0%) | 14 (63.6%) | 0.0159 |
| Heartburn, n (%) | 5 (100.0%) | 3 (13.6%) | 0.0007 |
| Regurgitation, n (%) | 4 (80.0%) | 3 (13.6%) | 0.0089 |
| Dysphagia, n (%) | 1 (20.0%) | 6 (27.3%) | 1.0000 |
| Freedom from PPI, n (%) | 0 (0.0%) | 18 (81.8%) | 0.0016 |
| DeMeester score, median (IQR)* | 45.4 (20.6–126) | 6.4 (3.5–20.1) | 0.0398 |
| pH normalization, n (%)* | 0 (0.0%) | 7 (77.8%) | 0.0455 |
*3 GERD and 9 Dysphagia group patients underwent post-removal Bravo
Impact of ARS at the time of removal
ARS was performed in 4 (44.4%) patients who underwent removal for GERD and 9 (29.0%) patients who underwent removal for dysphagia. The difference in symptomatic outcomes between groups who underwent device removal with and without additional ARS at the time of removal are shown in Table 5. Compared to patients who underwent ARS at removal, patients in the removal for GERD group who refused ARS had significantly lower rates of complete typical GERD symptom resolution (0.0 vs. 75.0%, p = 0.0476), due to higher rates of heartburn (100.0 vs 25.0%, p = 0.0476) and regurgitation (80.0 vs 0.0%, p = 0.0476). All and only patients who achieved complete symptom resolution achieved freedom from PPI in the removal for GERD group. ARS at the time of removal for dysphagia did not significantly impact post-removal symptoms or freedom from PPIs.
Table 5.
Impact of ARS at removal on post-removal symptoms
| Removal with ARS | Removal without ARS | p-value | ||
|---|---|---|---|---|
| (n = 4) | (n = 5) | |||
| Removal for recurrent GERD | Complete resolution, n (%) | 3 (75.0%) | 0 (0.0%) | 0.0476 |
| Heartburn, n (%) | 1 (25.0%) | 5 (100.0%) | 0.0476 | |
| Regurgitation, n (%) | 0 (0.0%) | 4 (80.0%) | 0.0476 | |
| Dysphagia, n (%) | 1 (25.0%) | 1 (20.0%) | 1.0000 | |
| Freedom from PPI, n (%) | 3 (75.0%) | 0 (0.0%) | 0.0476 | |
| (n = 9) | (n = 22) | |||
| Removal for persistent dysphagia | Complete resolution, n (%) | 7 (77.8%) | 14 (63.6%) | 0.6770 |
| Heartburn, n (%) | 1 (11.1%) | 3 (13.6%) | 1.0000 | |
| Regurgitation, n (%) | 0 (0.0%) | 3 (13.6%) | 0.5375 | |
| Dysphagia, n (%) | 1 (11.1%) | 6 (27.3%) | 0.6395 | |
| Freedom from PPI, n (%) | 6 (66.7%) | 18 (81.8%) | 0.3841 | |
In the removal for dysphagia group, post-removal pH monitoring was performed in a subset of 9 patients who underwent removal without ARS and 5 patients who received ARS at the time of removal. Median (IQR) DeMeester score in the removal for dysphagia with ARS group was 2.1 (0.8–6.3) and pH normalization was achieved by 5 (100.0%) patients, compared to a DeMeester score of 6.4 (3.9–13.7) and normalization rate of 7(77.8%) in the removal for dysphagia without ARS group. These DeMeester scores and pH normalization rates were not significantly different (p = 0.0605 & p = 0.2534, respectfully).
Dysphagia after removal
There were 7 (22.6%) patients in the removal for dysphagia group who continued to report dysphagia after device removal and underwent post-removal manometry. Analysis of their baseline, post-implant, and post-removal manometry characteristics was performed. There was a significant difference between the median (IQR) baseline and post-implant IRP [8.7 (8.4–10.0) vs. 19.7 (19.0–20.4), p = 0.0015], and the median baseline and post-removal IRP [8.7 (8.4–10.0) vs. 16.1 (11.7–22.6), p = 0.0025], but not between the post-implant and post-removal IRP (p = 0.2691) (Fig. 1). Additionally, there was a significant increase in median (IQR) percent incomplete bolus clearance from a baseline of 0.0% (0.0–20.0) to 70.0% (40.0–70.0) after implant (p = 0.0087), which then significantly decreased to 10.0% (0.0–30.0) after removal (p = 0.0465). There was no significant difference between baseline and post-removal bolus clearance (p = 0.5265). No other manometric features were significantly different.
Fig. 1.
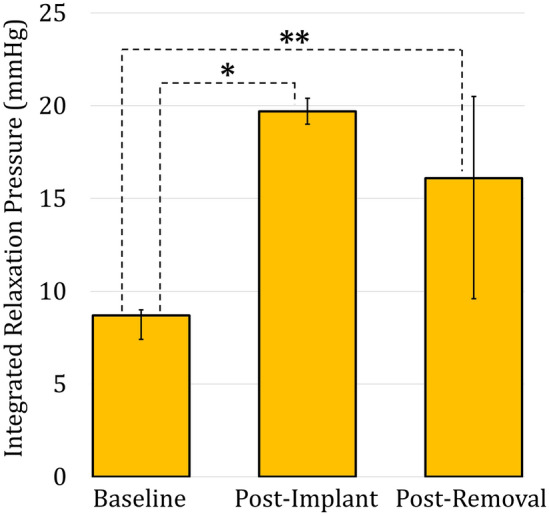
Median and interquartile range (error bars) of the integrated relaxation pressures at baseline, post-implantation, and post-removal among 7 patients with persistent dysphagia after device removal. There was a significant increase from baseline to post-implant (p = 0.0015) and baseline to post-removal (p = 0.0025)
Discussion
The goal of any anti-reflux surgery is to prevent retrograde flow of gastric contents by restoring the competency of the gastro-esophageal junction (GEJ). If postoperative resistance at the GEJ is too high, both retrograde and anterograde flow stops, and the patient suffers from dysphagia [16, 17]. If the GEJ competency has not been restored adequately, the surgery is ineffective and reflux symptoms persist [17]. The ideal management of these postoperative complications should target the underlying mechanism of failure. In the present study, we reported our experience with device removal after MSA due to the complications of recurrent GERD symptoms and persistent dysphagia. Our major finding was that clinical outcomes were dependent on the indication for device removal. Patients who underwent removal for dysphagia had higher rates of heartburn resolution, regurgitation resolution and pH normalization both after implant and removal. Additionally, these patients had comparatively excellent outcomes with or without ARS at the time of removal. By contrast, patients with recurrent GERD symptoms after MSA who refused ARS at the time of removal had significantly worse outcomes, with all patients failing to achieve complete symptom resolution, freedom from PPIs or pH normalization. These findings suggest that surgical management should be tailored to the indication for device removal.
Heartburn, regurgitation and abnormal esophageal acid exposure significantly improved after device implantation in the removal for dysphagia group, suggesting an adequate increase in EGJ resistance, preventing reflux. Similarly, previous studies of dysphagia after fundoplication have shown that reflux symptom control is not diminished by postoperative dysphagia. [18] Studies of patients with dysphagia after MSA have even shown higher rates of pH normalization than those without dysphagia. [19] Further studies have shown that as mean pressure across the GEJ rises, so does severity of dysphagia. [20] These findings suggest that persistent dysphagia after MSA is the result of a supra-competent LES that prevents both retrograde and anterograde flow.
Early dysphagia following MSA is common, but usually self-limiting. [19] Dietary exercises consisting of small bites of solid food every waking hour for the first several weeks after surgery maintains a degree of elasticity at the GEJ and prevents stricture as scar tissue forms and contracts [11]. Early dilation after MSA can trigger an inflammatory reaction in the soft tissue surrounding the device, exacerbating scarification, and should be avoided. [21] Dysphagia lasting more than 8 weeks occurs in up to 19% of cases, and may correspond with the development of a robust capsule around the MSA [22]. Therefore, at our institution consideration for dilation is delayed until at least 8-weeks after MSA. Following dilation under fluoroscopic guidance patients are prescribed a seven-day course of twice daily 20 mg prednisone and dietary modifications to minimize the inflammatory response. After three endoscopic dilations without durable improvement, device removal should be considered.
Persistent post-operative dysphagia, refractory to diet and multiple dilations, is a complex multi-faceted problem involving esophageal motility, device size, host response to implant, and individual subjective perception of dysphagia, all of which warrant further esophageal testing [23]. Endoscopy may reveal normal resistance at the EGJ, but marked resistance may indicate the formation of robust scar tissue surrounding the EGJ. Stasis esophagitis or candidiasis are possible as well. Barium swallow may reveal EGJ narrowing, delayed transit, and an elevated contrast column. Following MSA, HRM commonly shows an elevated IRP, elevated intrabolus pressure, and a compensatory increase in esophageal body contractions (Fig. 2). [24] However, in patients with debilitating post-operative dysphagia, these findings are often more pronounced and HRM can reveal impaired peristaltic progression with poor bolus clearance. There have even been reports of pseudoachalasia following MSA from this increase in the GEJ outflow resistance [25].
Fig. 2.
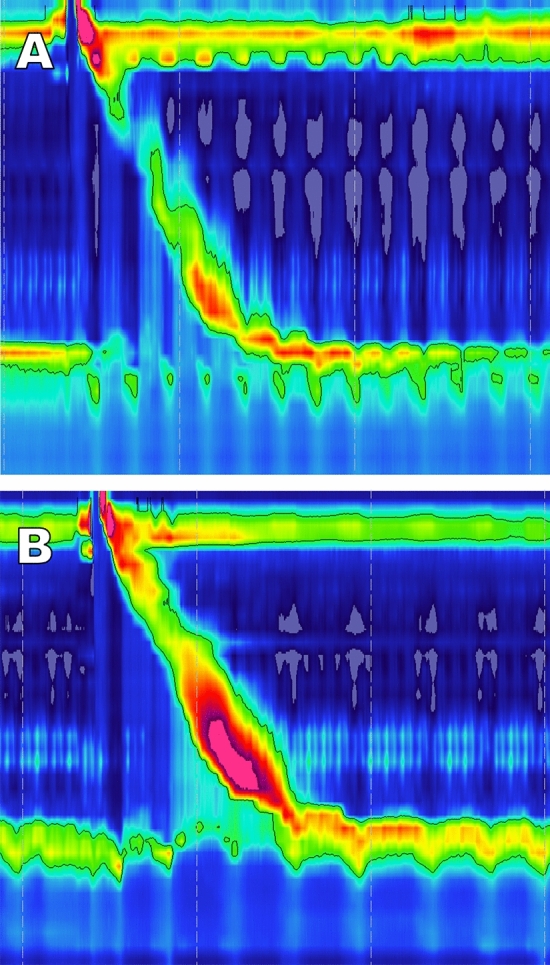
HRM topographic plot before (A) and after MSA implantation (B), but before removal in a patient who subsequently underwent device removal for dysphagia. At baseline (A) the patient has adequate distal contractile integral (DCI) with complete bolus clearance (not shown). There is complete LES relaxation and evidence of some degree of crural-LES separation, indicating a hiatal hernia. Following device implantation (B) the topographic plot shows an elevated intrabolus pressure (iBP) and integrated relaxation pressure (IRP) and a compensatory increase in esophageal body contractility
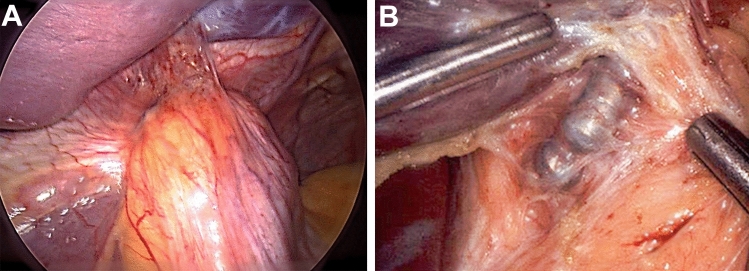
In the removal for dysphagia group, the post-implant improvements in heartburn, regurgitation and pH-normalization persisted after device removal, and were unaffected by additional ARS, suggesting that removal alone is adequate. Tatum et al. found similar results in their study of removal among 435 patients who had undergone MSA. They found that 77.8% of patients who required device removal for dysphagia did not require additional ARS. [10] These findings suggest that device removal does not return EGJ pressure to baseline; some degree of LES augmentation remains. The reason for this residual augmentation is likely due to the tissue reaction to the titanium beads of the MSA device. Studies of orthopedic and dental implants have demonstrated that titanium biologic implants stimulate a foreign body response, which walls off the titanium with a thick fibrous capsule [26–29]. Our experience with device removal demonstrated that a similar fibrous capsule forms around the gastroesophageal junction (Fig. 3). Initially, MSA works by increasing external augmentation at the LES with circumferential titanium magnets. However, as the capsule forms around the device, it further augments the barrier function of the lower esophageal sphincter. [30] When a MSA device is removed, the capsule remains, and the forces exerted by it can provide enough residual sphincter augmentation to control reflux and balance the EGJ resistance.
Fig. 3.
Dense fibrotic capsule surrounding the MSA. (A) Fibrosis completely encapsulated the titanium beads. (B) The capsule can be thick and can hold substantial tension even after partial opening
The addition of an anti-reflux surgery at the time of removal acts to counterbalance the expected decrease in EGJ resistance and increase in reflux caused by removing the augmentation. However, among those who underwent removal for dysphagia, the residual capsule appears to be enough to balance EGJ resistance, and additional anti-reflux surgery is unnecessary. By contrast when EGJ resistance after implantation was inadequate, as in our recurrent GERD group, removal alone resulted in failure to control symptoms, free patients from PPI use, or normalize distal esophageal pH. Therefore, additional ARS at the time of removal should be strongly recommended in these patients.
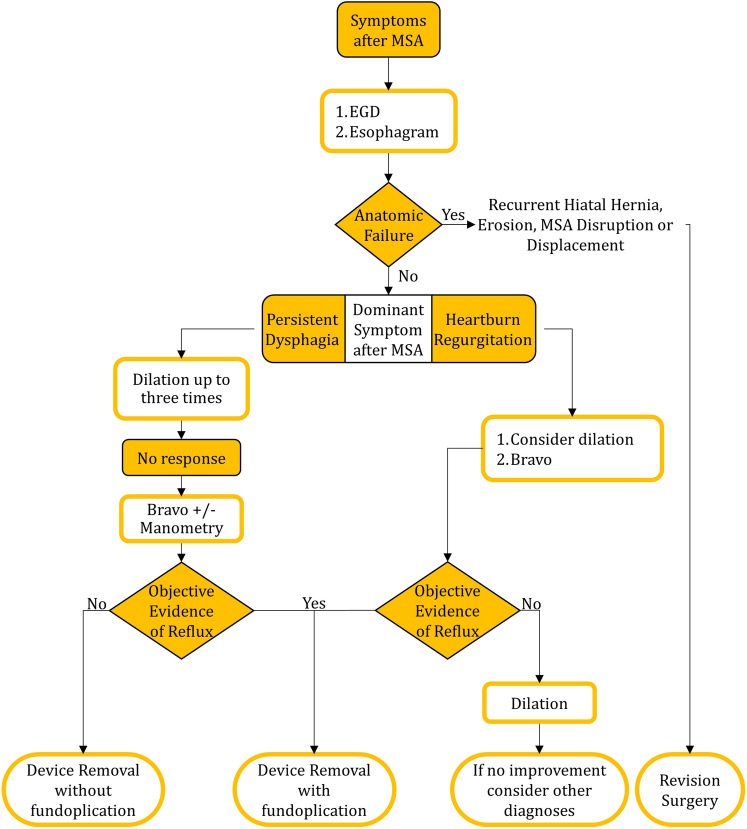
Almost half of patients who underwent device removal for persistent GERD symptoms reported dysphagia, and 29% of patients who underwent removal for dysphagia reported heartburn or regurgitation. Therefore, symptoms alone are insufficient to tailor surgical management. Patients being considered for device removal should undergo objective foregut evaluation. The assessment should begin with a revaluation of the postoperative anatomic integrity. A barium esophagram and EGD can identify recurrent hiatal hernia or device erosion, disruption, or displacement [31, 32]. In a symptomatic patient, any of these findings is a clear indication of failure and need for surgical revision [32, 33]. When the repair is anatomically intact endoscopic evaluation for esophagitis and ambulatory pH monitoring become essential. If worsening esophagitis or abnormal distal esophageal acid exposure are discovered, the initial augmentation has failed and additional ARS is needed. If a patient undergoing removal for dysphagia has also failed from a reflux control standpoint, the construction of a floppy Nissen or partial fundoplication should be considered. By contrast, in a patient with dysphagia with normal distal esophageal acid exposure, high IRP and poor bolus clearance on HRM can aid in identifying an elevated resistance at the GEJ [34, 35]. Based on the findings of the present study, device removal without additional ARS is adequate for these patients. Sustained non-dysphagia reflux symptoms despite unremarkable objective results can be due to a number of conditions including: non-acidic reflux, esophageal hypersensitivity, and sensations secondary to esophageal wall distension. Objective tests prior to device removal are crucial as the comparison to preoperative values, and the correlation of symptoms with objective findings, greatly assists with surgical decision-making and patient counseling. Therefore, we propose a treatment paradigm for the management of heartburn, regurgitation and/or dysphagia after MSA as demonstrated in Fig. 4.
Fig. 4.
Proposed management paradigm for patients complaining of dysphagia or inadequate relief of their reflux symptoms after MSA
Limitations of this study include its retrospective nature, small sample size, and variable post-implant and post-removal workup. Not all the patients underwent pH monitoring and manometry following device implant and removal. Further studies with larger populations and standardized objective testing regimens are necessary to confirm our findings.
Conclusion
We found that device removal after MSA for persistent dysphagia or insufficient reflux control is necessary in 5.1% of implantations. Patients who had undergone removal for dysphagia had excellent symptom resolution and objective pH normalization, regardless of whether they had additional anti-reflux surgery at the time of removal. By contrast, patients who refused ARS at time of device removal for insufficient reflux control had significantly worse outcomes on all measures. Objective testing prior to device removal is necessary to identify the underlying mechanism MSA failure, and surgical management of these patients should be tailored to the indication for device removal.
Acknowledgements
None.
Declarations
Disclosure
SA and BJ: serve on the scientific advisory board of Johnson and Johnson and receive a consulting fee. SE, KS, TH and PZ have no conflicts of interest or financial disclosures. Authors SE, KS, SA, TH, PZ, BAJ have no conflicts of interest or financial ties to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lipham JC, DeMeester TR, Ganz RA, Bonavina L, Saino G, Dunn DH, Fockens P, Bemelman W. The LINX(R) reflux management system: confirmed safety and efficacy now at 4 years. Surg Endosc. 2012;26:2944–2949. doi: 10.1007/s00464-012-2289-1. [DOI] [PubMed] [Google Scholar]
- 2.Dunn CP, Zhao J, Wang JC, Patel TA, Putnam LR, Eka A, Houghton CC, Bildzukewicz NA, Lipham JC. Magnetic sphincter augmentation with hiatal hernia repair: long term outcomes. Surg Endosc. 2021;35:5607–5612. doi: 10.1007/s00464-020-08063-9. [DOI] [PubMed] [Google Scholar]
- 3.Bonavina L, DeMeester T, Fockens P, Dunn D, Saino G, Bona D, Lipham J, Bemelman W, Ganz RA. Laparoscopic sphincter augmentation device eliminates reflux symptoms and normalizes esophageal acid exposure: one- and 2-year results of a feasibility trial. Ann Surg. 2010;252:857–862. doi: 10.1097/SLA.0b013e3181fd879b. [DOI] [PubMed] [Google Scholar]
- 4.Ayazi S, Zheng P, Zaidi AH, Chovanec K, Salvitti M, Newhams K, Hoppo T, Jobe BA. Clinical outcomes and predictors of favorable result after laparoscopic magnetic sphincter augmentation: single-institution experience with more than 500 patients. J Am Coll Surg. 2020;230:733–743. doi: 10.1016/j.jamcollsurg.2020.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Zadeh J, Andreoni A, Treitl D, Ben-David K. Spotlight on the Linx Reflux management system for the treatment of gastroesophageal reflux disease: evidence and research. Med Devices (Auckl) 2018;11:291–300. doi: 10.2147/MDER.S113679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds JL, Zehetner J, Wu P, Shah S, Bildzukewicz N, Lipham JC. Laparoscopic magnetic sphincter augmentation vs laparoscopic nissen fundoplication: a matched-pair analysis of 100 patients. J Am Coll Surg. 2015;221:123–128. doi: 10.1016/j.jamcollsurg.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 7.Ayazi S, Zheng P, Zaidi AH, Chovanec K, Chowdhury N, Salvitti M, Komatsu Y, Omstead AN, Hoppo T, Jobe BA. Magnetic sphincter augmentation and postoperative dysphagia: characterization, clinical risk factors, and management. J gastroint surg. 2019;24(1):39–49. doi: 10.1007/s11605-019-04331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkham EN, Main BG, Jones KJB, Blazeby JM, Blencowe NS. Systematic review of the introduction and evaluation of magnetic augmentation of the lower oesophageal sphincter for gastro-oesophageal reflux disease. Br J Surg. 2020;107:44–55. doi: 10.1002/bjs.11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asti E, Siboni S, Lazzari V, Bonitta G, Sironi A, Bonavina L. Removal of the magnetic sphincter augmentation device: surgical technique and results of a single-center cohort study. Ann Surg. 2017;265:941–945. doi: 10.1097/SLA.0000000000001785. [DOI] [PubMed] [Google Scholar]
- 10.Tatum JM, Alicuben E, Bildzukewicz N, Samakar K, Houghton CC, Lipham JC. Removing the magnetic sphincter augmentation device: operative management and outcomes. Surg Endosc. 2019;33:2663–2669. doi: 10.1007/s00464-018-6544-y. [DOI] [PubMed] [Google Scholar]
- 11.Bonavina L, Saino G, Lipham JC, DeMeester TR. LINX® Reflux management system in chronic gastroesophageal reflux: a novel effective technology for restoring the natural barrier to reflux. Ther Adv Gastroenterol. 2013;6:261–268. doi: 10.1177/1756283X13486311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganz RA, Edmundowicz SA, Taiganides PA, Lipham JC, Smith CD, DeVault KR, Horgan S, Jacobsen G, Luketich JD, Smith CC. Long-term outcomes of patients receiving a magnetic sphincter augmentation device for gastroesophageal reflux. Clin Gastroenterol Hepatol. 2016;14:671–677. doi: 10.1016/j.cgh.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 13.Velanovich V. The development of the GERD-HRQL symptom severity instrument. Dis esophagus. 2007;20:130–134. doi: 10.1111/j.1442-2050.2007.00658.x. [DOI] [PubMed] [Google Scholar]
- 14.Ayazi S, Lipham JC, Portale G, Peyre CG, Streets CG, Leers JM, DeMeester SR, Banki F, Chan LS, Hagen JA. Bravo catheter-free pH monitoring: normal values, concordance, optimal diagnostic thresholds, and accuracy. Clin Gastroenterol Hepatol. 2009;7:60–67. doi: 10.1016/j.cgh.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Ganz RA. A modern magnetic implant for gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2017;15:1326–1337. doi: 10.1016/j.cgh.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Wilshire CL, Niebisch S, Watson TJ, Litle VR, Peyre CG, Jones CE, Peters JH. Dysphagia postfundoplication: more commonly hiatal outflow resistance than poor esophageal body motility. Surgery. 2012;152:584–594. doi: 10.1016/j.surg.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Ayazi S, Grubic AD, Zheng P, Zaidi AH, Schwameis K, Alleyne AC, Myers BM, Omstead AN, Jobe BA. Measurement of outflow resistance imposed by magnetic sphincter augmentation: defining normal values and clinical implication. Surg Endosc. 2021;35:5787–5795. doi: 10.1007/s00464-020-08068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makris KI, Cassera MA, Kastenmeier AS, Dunst CM, Swanström LL. Postoperative dysphagia is not predictive of long-term failure after laparoscopic antireflux surgery. Surg Endosc. 2012;26:451–457. doi: 10.1007/s00464-011-1898-4. [DOI] [PubMed] [Google Scholar]
- 19.Ayazi S, Zheng P, Zaidi AH, Chovanec K, Chowdhury N, Salvitti M, Komatsu Y, Omstead AN, Hoppo T, Jobe BA. Magnetic sphincter augmentation and postoperative dysphagia: characterization, clinical risk factors, and management. J Gastrointest Surg. 2019;24:39–49. doi: 10.1007/s11605-019-04331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blom D, Peters JH, DeMeester TR, Crookes PF, Hagan JA, DeMeester SR, Bremner C. Physiologic mechanism and preoperative prediction of new-onset dysphagia after laparoscopic Nissen fundoplication. J Gastrointest Surg. 2002;6:22–28. doi: 10.1016/S1091-255X(01)00051-8. [DOI] [PubMed] [Google Scholar]
- 21.Ayazi S, Zheng P, Zaidi AH, Chovanec K, Chowdhury N, Salvitti M, Komatsu Y, Omstead AN, Hoppo T, Jobe BA. Magnetic sphincter augmentation and postoperative dysphagia: characterization, clinical risk factors, and management. J Gastrointest Surg. 2020;24:39–49. doi: 10.1007/s11605-019-04331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asti E, Aiolfi A, Lazzari V, Sironi A, Porta M, Bonavina L. Magnetic sphincter augmentation for gastroesophageal reflux disease: review of clinical studies. Updat Surg. 2018;70:323–330. doi: 10.1007/s13304-018-0569-6. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg JA, Stefanova DI, Reyes FV, Edelmuth RC, Harik L, Thiesmeyer JW, Egan CE, Palacardo F, Liu M, Christos P. Evaluation of post-operative dysphagia following anti-reflux surgery. Surg Endosc. 2022;36:5456–5466. doi: 10.1007/s00464-021-08888-y. [DOI] [PubMed] [Google Scholar]
- 24.Ayazi S, Schwameis K, Zheng P, Newhams K, Myers BM, Grubic AD, Hoppo T, Jobe BA. The impact of magnetic sphincter augmentation (MSA) on esophagogastric junction (EGJ) and esophageal body physiology and manometric characteristics. Ann surg. 2021 doi: 10.1097/SLA.0000000000005239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwameis K, Ayazi S, Zaidi AH, Hoppo T, Jobe BA. Development of pseudoachalasia following magnetic sphincter augmentation (MSA) with restoration of peristalsis after endoscopic dilation. Clin J Gastroenterol. 2020;13:697–702. doi: 10.1007/s12328-020-01140-5. [DOI] [PubMed] [Google Scholar]
- 26.Kaur M, Singh K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater Sci Eng C Mater Biol Appl. 2019;102:844–862. doi: 10.1016/j.msec.2019.04.064. [DOI] [PubMed] [Google Scholar]
- 27.Suska F, Emanuelsson L, Johansson A, Tengvall P, Thomsen P. Fibrous capsule formation around titanium and copper. J Biomed Mater Res A. 2008;85:888–896. doi: 10.1002/jbm.a.31575. [DOI] [PubMed] [Google Scholar]
- 28.Long PH. Medical devices in orthopedic applications. Toxicol Pathol. 2008;36:85–91. doi: 10.1177/0192623307310951. [DOI] [PubMed] [Google Scholar]
- 29.Eger M, Hiram-Bab S, Liron T, Sterer N, Carmi Y, Kohavi D, Gabet Y. Mechanism and prevention of titanium particle-induced inflammation and osteolysis. Front Immunol. 2018;9:2963. doi: 10.3389/fimmu.2018.02963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rona KA, Reynolds J, Schwameis K, Zehetner J, Samakar K, Oh P, Vong D, Sandhu K, Katkhouda N, Bildzukewicz N, Lipham JC. Efficacy of magnetic sphincter augmentation in patients with large hiatal hernias. Surg Endosc. 2017;31:2096–2102. doi: 10.1007/s00464-016-5204-3. [DOI] [PubMed] [Google Scholar]
- 31.Buckley FP, 3rd, Bell RCW, Freeman K, Doggett S, Heidrick R. Favorable results from a prospective evaluation of 200 patients with large hiatal hernias undergoing LINX magnetic sphincter augmentation. Surg endosc. 2017;32(4):1762–1768. doi: 10.1007/s00464-017-5859-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jobe BA, Kahrilas PJ, Vernon AH, Sandone C, Gopal DV, Swanstrom LL, Aye RW, Hill LD, Hunter JG. Endoscopic appraisal of the gastroesophageal valve after antireflux surgery. American J Gastroenterol. 2004;99:233–243. doi: 10.1111/j.1572-0241.2004.04042.x. [DOI] [PubMed] [Google Scholar]
- 33.Rona KA, Tatum JM, Zehetner J, Schwameis K, Chow C, Samakar K, Dobrowolsky A, Houghton CC, Bildzukewicz N, Lipham JC. Hiatal hernia recurrence following magnetic sphincter augmentation and posterior cruroplasty: intermediate-term outcomes. Surg Endosc. 2018;32:3374–3379. doi: 10.1007/s00464-018-6059-6. [DOI] [PubMed] [Google Scholar]
- 34.Ayazi S. Esophageal manometry testing and anti-reflux surgery: the preoperative necessity and prognostic utility. Foregut. 2021;1:216–226. doi: 10.1177/26345161211044448. [DOI] [Google Scholar]
- 35.Alicuben ET, Bell RC, Jobe BA, Buckley F, Daniel Smith C, Graybeal CJ, Lipham JC. Worldwide experience with erosion of the magnetic sphincter augmentation device. J Gastrointest Surg. 2018;22:1442–1447. doi: 10.1007/s11605-018-3775-0. [DOI] [PubMed] [Google Scholar]