Figure 4. Crystallographic studies of SIRT3 in complex with CCNE2K348la.
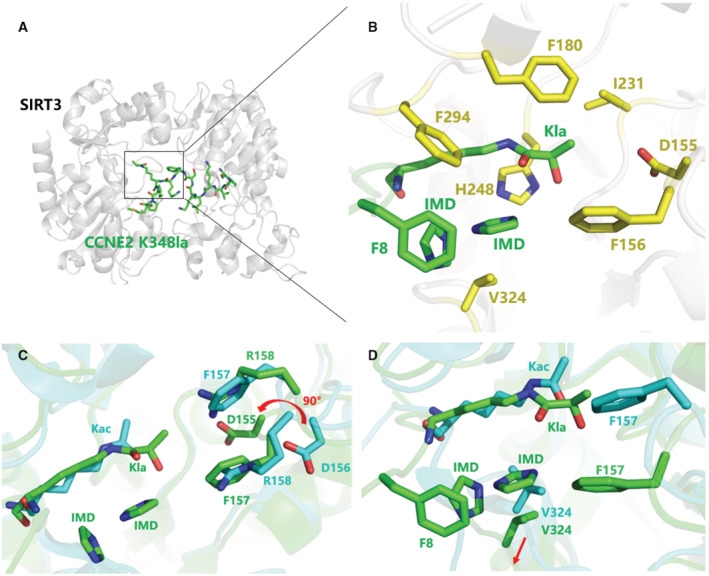
- The overall complex structure of SIRT3 (gray) interaction with CCNE2K348la peptide (green).
- The catalytic pocket of SIRT3 catalyzing the Kla group. Several residues (yellow) consist of the pocket to accommodate the Kla group, nearby Phe8 and two imidazole molecules (green).
- The structural superimposition of SIRT3/CCNE2Kla (green) with SIRT3/AceCS2Kac (PDB 3GLR, Cyan). The reconfiguration of the residues (D156, F157, R158) in the catalytic pocket facilitates the Kla group binding compared to those conformations when SIRT3 binds with Kac.
- A conformational change of the residue V324 facilitates the formation of a pocket together with F8 from CCNE2 peptide and re‐organizes F157 to accommodate two imidazoles bridging the interaction of SIRT3 with the peptide substrate.
