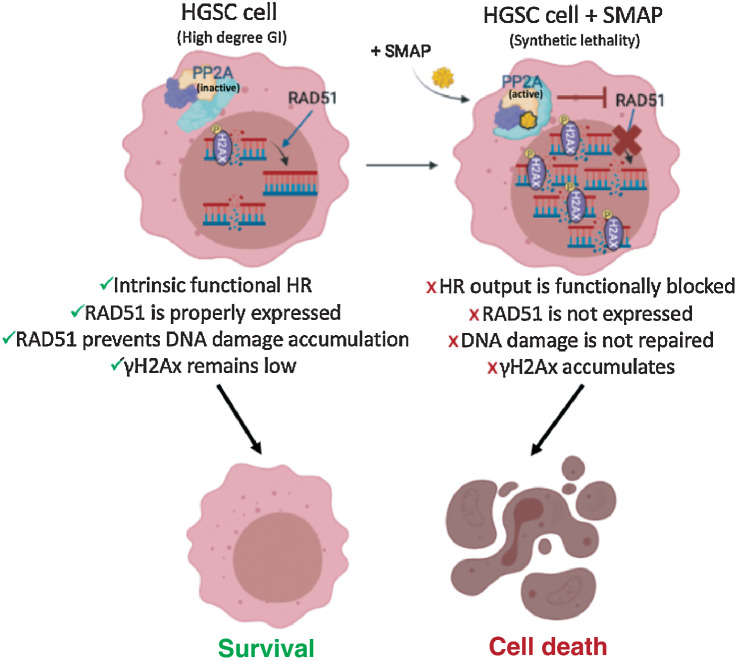
Figure 7.
RAD51 inhibition leads to the chronic accumulation of γH2Ax and DNA errors inherent from genomic instability (GI), an ovarian cancer trait. HGSC cells harbor a high degree of GI that results in small nicks and breaks in the DNA over time. Sensor proteins such as γH2Ax are recruited to the site of damage to prevent DNA degradation and remain in check until a repair mechanism is triggered. The minimally functional HR machinery is activated and prompts the recruitment of the main downstream effector protein, RAD51, which by keeping those damaging signals at bay, allows cancer cells to survive, even if with relatively higher baseline levels of γH2Ax when compared with normal cells. For this reason, ovarian cancer cells are known to functionally tolerate and survive high baseline levels of DNA damage while avoiding apoptotic cues (left). When SMAP-061 is added to the cell, PP2A's antitumor activity is functionally restored, and synthetic lethality is induced (right). RAD51 expression is inhibited by PP2A, preventing the HR output to be efficiently propagated. This results in the incapacity of the cells to repair their inherent DNA damage, thereby leading to the accumulation of γH2Ax signals in the nucleus, and ultimately, triggering apoptosis (schematic created with BioRender.com).