Fig. 2. Scope of the photoenzymatic cross-electrophile couplings.
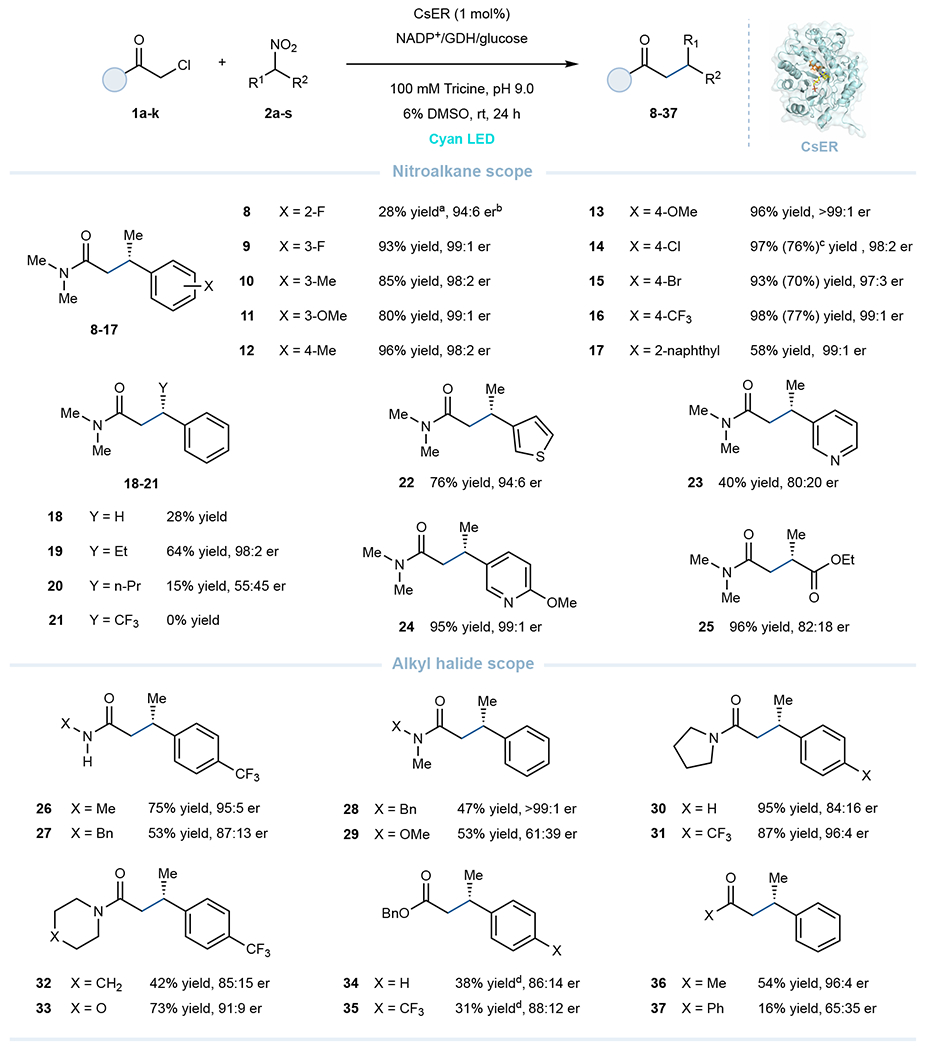
Reaction conditions: α-chloro carbonyl substrate (10 μmol, 2 equiv), nitroalkane (5 μmol, 1 equiv), GDH-105 (0.3 mg), NADP+ (0.05 μmol, 1 mol%), glucose (25 μmol) and purified ‘ene’-reductases (0.05 μmol, 1 mol% based on nitroalkane) in tricine buffer (100 mM, pH 9.0), with 6% DMSO as cosolvent, final total volume is 800 μL. Reaction mixtures were irradiated with cyan LEDs under anaerobic conditions at room temperature for 24 h. a Yields determined via LCMS relative to an internal standard (TBB). b Enantiomeric ratio (er) refers to as the ratio of (S)- to (R)-enantiomer, er determined by HPLC on a chiral stationary phase. c Isolated yields were based on 0.10 mmol-scale reaction. d 3 equiv of α-bromo ester (15 μmol) were used.
