This prognostic study assesses if polygenic risk scores for coronary heart disease and acute ischemic stroke predict incident atherosclerotic cardiovascular disease events.
Key Points
Question
Do polygenic risk scores (PRSs) for coronary heart disease and acute ischemic stroke predict incident atherosclerotic cardiovascular disease (ASCVD) events?
Findings
In an ancestrally diverse, primary prevention sample of almost 80 000 veterans observed for up to 7 years, PRSs were significantly associated with incident myocardial infarction, acute ischemic stroke, and cardiovascular death. Discrimination was modest overall but greater among women and younger participants.
Meaning
PRSs provide modest incremental benefit for ASCVD risk stratification in a general midlife and older age population over and above traditional risk factors.
Abstract
Importance
Primary prevention of atherosclerotic cardiovascular disease (ASCVD) relies on risk stratification. Genome-wide polygenic risk scores (PRSs) are proposed to improve ASCVD risk estimation.
Objective
To determine whether genome-wide PRSs for coronary artery disease (CAD) and acute ischemic stroke improve ASCVD risk estimation with traditional clinical risk factors in an ancestrally diverse midlife population.
Design, Setting, and Participants
This was a prognostic analysis of incident events in a retrospectively defined longitudinal cohort conducted from January 1, 2011, to December 31, 2018. Included in the study were adults free of ASCVD and statin naive at baseline from the Million Veteran Program (MVP), a mega biobank with genetic, survey, and electronic health record data from a large US health care system. Data were analyzed from March 15, 2021, to January 5, 2023.
Exposures
PRSs for CAD and ischemic stroke derived from cohorts of largely European descent and risk factors, including age, sex, systolic blood pressure, total cholesterol, high-density lipoprotein (HDL) cholesterol, smoking, and diabetes status.
Main Outcomes and Measures
Incident nonfatal myocardial infarction (MI), ischemic stroke, ASCVD death, and composite ASCVD events.
Results
A total of 79 151 participants (mean [SD] age, 57.8 [13.7] years; 68 503 male [86.5%]) were included in the study. The cohort included participants from the following harmonized genetic ancestry and race and ethnicity categories: 18 505 non-Hispanic Black (23.4%), 6785 Hispanic (8.6%), and 53 861 non-Hispanic White (68.0%) with a median (5th-95th percentile) follow-up of 4.3 (0.7-6.9) years. From 2011 to 2018, 3186 MIs (4.0%), 1933 ischemic strokes (2.4%), 867 ASCVD deaths (1.1%), and 5485 composite ASCVD events (6.9%) were observed. CAD PRS was associated with incident MI in non-Hispanic Black (hazard ratio [HR], 1.10; 95% CI, 1.02-1.19), Hispanic (HR, 1.26; 95% CI, 1.09-1.46), and non-Hispanic White (HR, 1.23; 95% CI, 1.18-1.29) participants. Stroke PRS was associated with incident stroke in non-Hispanic White participants (HR, 1.15; 95% CI, 1.08-1.21). A combined CAD plus stroke PRS was associated with ASCVD deaths among non-Hispanic Black (HR, 1.19; 95% CI, 1.03-1.17) and non-Hispanic (HR, 1.11; 95% CI, 1.03-1.21) participants. The combined PRS was also associated with composite ASCVD across all ancestry groups but greater among non-Hispanic White (HR, 1.20; 95% CI, 1.16-1.24) than non-Hispanic Black (HR, 1.11; 95% CI, 1.05-1.17) and Hispanic (HR, 1.12; 95% CI, 1.00-1.25) participants. Net reclassification improvement from adding PRS to a traditional risk model was modest for the intermediate risk group for composite CVD among men (5-year risk >3.75%, 0.38%; 95% CI, 0.07%-0.68%), among women, (6.79%; 95% CI, 3.01%-10.58%), for age older than 55 years (0.25%; 95% CI, 0.03%-0.47%), and for ages 40 to 55 years (1.61%; 95% CI, −0.07% to 3.30%).
Conclusions and Relevance
Study results suggest that PRSs derived predominantly in European samples were statistically significantly associated with ASCVD in the multiancestry midlife and older-age MVP cohort. Overall, modest improvement in discrimination metrics were observed with addition of PRSs to traditional risk factors with greater magnitude in women and younger age groups.
Introduction
Atherosclerotic cardiovascular disease (ASCVD) is a tremendous source of morbidity and mortality globally.1 Primary prevention with statin and aspirin therapy can significantly decrease this burden; however, estimating patient risk is a key first step to identifying patients who would benefit. Despite improvements in the last decade, current clinical prediction models misclassify a considerable number of individuals, resulting in overtreatment or undertreatment of ASCVD risk.2,3
Polygenic risk scores (PRSs) have been proposed to improve CVD risk stratification. Advances in statistical methods, computational capability, and the size of genome-wide association studies (GWASs) for discovery have improved the performance of PRSs in discriminating disease cases from controls and estimating risk of incident disease. Specifically, significant advances have occurred in the development and validation of PRSs for coronary artery disease (CAD), including their extension into populations of diverse genetic ancestry.4,5 Research and clinical efforts are now underway to evaluate the utility of PRSs in patient risk stratification and disease prevention.6,7,8 The advent of genetic research and promulgation of polygenic risk analyses across the globe has added a new perspective to CVD prevention.9,10,11
In current clinical practice in the US, standard of care includes using a risk calculator to estimate a patient’s absolute risk for ASCVD, a composite outcome including CAD events (nonfatal myocardial infarction, coronary heart disease death), and fatal or nonfatal stroke. Prior research has demonstrated that PRSs derived from CAD GWAS data may modestly improve the risk estimation achieved by clinical risk prediction models such as the Pooled Cohort Equations.2,12 However, few studies have examined how leveraging GWAS data from multiple ASCVD phenotypes, namely CAD and acute ischemic stroke, contributes to ASCVD risk estimation through the addition of PRSs and whether the degree of improvement in risk prediction varies by ancestry.4
The hypothesis tested was that incorporating PRSs for both CAD and ischemic stroke into a clinical ASCVD risk model improves risk estimation compared with the clinical model alone. Data were analyzed from the Million Veteran Program (MVP) within the Veterans Health Administration (VHA). The MVP is a large, national, ancestrally diverse longitudinal cohort study with genetic, survey, and electronic medical record data.13 First, previously published CAD and ischemic stroke PRSs derived from cohorts of largely European descent were assessed for their ability to predict individual and composite ASCVD outcomes in a multiancestry population. Second, the performance for predicting ASCVD events from traditional risk factor models was compared against PRS models and models including both traditional risk factors and PRSs.
Methods
Study Population
This prognostic study was approved by the VA Central Institutional Review Board. All participants provided written informed consent. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guidelines were followed.
The study sample was drawn from MVP participants who were genotyped and had at least 1 outpatient lipid measurement in the year before enrollment. To create a primary ASCVD prevention cohort, patients were excluded for history of myocardial infarction (MI), revascularization, ischemic stroke, or statin use at baseline.14 Additionally, participants with serious comorbid conditions were excluded because a traditional ASCVD risk model would inadequately estimate risk in these patient populations; these conditions included HIV positivity, chronic kidney disease, liver disease or hepatitis, cancer (other than nonmelanoma skin cancer), schizophrenia, dementia, and amputation.14 Participants were categorized into harmonized ancestry and race and ethnicity (HARE) groups. As described previously, the HARE algorithm uses a machine learning model to predict self-identified race and ethnicity (SIRE) from principal components of genetic ancestry and was developed to facilitate ancestry-specific GWAS in the MVP.15 The HARE algorithm was trained on SIRE groups based on participant responses to the MVP baseline survey questions “Are you Spanish, Hispanic, or Latino?” and “What is your race (American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or Other Pacific Islander, White, or other)?” HARE was defined for the 4 largest race and ethnicity groups: non-Hispanic Asian, non-Hispanic Black, Hispanic, and non-Hispanic White (abbreviated as Asian, Black, Hispanic, and White, respectively). The Asian group was excluded in the present analyses due to limited ASCVD data (<100 incident events). Although SIRE was previously used to train the HARE algorithm, it was not used in these analyses. Agreement between HARE and genetically informed ancestry (GIA) classification was very high for all ancestries except the Admixed American ancestry (ie, continental American populations with different global proportions of European, Native American, and Sub-Saharan African ancestry), among whom 10% were classified by HARE as non-Hispanic White, and 90% were classified as Hispanic (eTable 1 in Supplement 1). This degree of overlap reflects recent admixture among Native American, European and African ancestral populations and is appropriate for ASCVD risk estimation.16 Given the high degree of concordance between HARE and GIA, no separate analyses were performed for GIA. Patient follow-up extended from MVP enrollment in January 1, 2011, until December 31, 2018.
Data Sources
Electronic health records were extracted from the national VA Corporate Data Warehouse.17 Outcomes ascertained in the VHA were supplemented with records from the Centers for Medicaid & Medicare Services (CMS) and National Death Index databases.18,19 Genetic variants in MVP were genotyped with an Applied Biosystems (formerly Affymetrix) microarray (Thermo Fisher Scientific).20
PRSs
Primary analyses used PRSs for ischemic stroke21 and CAD,22 which combined GWAS associations from European and non-European ancestries but were optimized in European cohorts. Sensitivity analyses in young adults (age <40 years) were conducted with PRSs from the UK Biobank (UKBB) for ischemic stroke and CAD23 to better match previous UK studies of age-related PRS trends. For variants not genotyped in MVP, dosages were imputed using the 1000 Genomes Project reference panel24 and African Genome Resources panel.25
Other Risk Factors
Patient characteristics were assessed at baseline, which was an outpatient VA clinic visit close to MVP enrollment date. Baseline diabetes status was defined as outpatient hemoglobin A1c level of at least 6.5% or any prescription for diabetes medication before enrollment plus 1 International Classification of Diseases, Ninth Revision (ICD-9) 250.xx (or equivalent Tenth Revision [ICD-10]) code in combination with a VA primary care visit or at least 2 total ICD codes. Smoking status (current, former, never) was ascertained from smoking-related health factors and ICD-9 codes for tobacco dependence using an algorithm previously validated in the VA.26 Statin therapy and antihypertensive therapy were defined as an active prescription for a relevant medication on the baseline date.14 Continuous clinical measures were averaged over all outpatient measures recorded in the preceding year. Medications and ICD codes used for inclusion/exclusion are available in a previous publication.14
Outcomes
Incident ischemic stroke and MI events were defined as the first occurrences of ICD-9, ICD-10, or CMS codes used by phenotyping algorithms that had been validated with expert medical record review in the VA.14,27,28 Incident ischemic stroke ICD codes included 433.x1, 434 (excluding 434.x0), 436, 437.0, 437.6, I63.xx9, I63.20, I63.22, I63.30, I63.40, I63.50, I63.59, I67.2, I67.6, and I67.89. Incident MI ICD codes included 410, 411.0, I21, and I22. ASCVD death was defined as any of the following ICD-10–Clinical Modification diagnosis codes as the primary cause of death in the National Death Index: I10, I11, I13, I16, I20 to I25, I46, I63, I67, I70, I74, I75, and G45.14 Composite ASCVD events included first occurrence of ischemic stroke, MI, or ASCVD death. Revascularization was defined as any percutaneous coronary intervention and coronary artery bypass graft (eTable 2 in Supplement 1). Follow-up time for each ASCVD outcome began at MVP enrollment date and ended at date of first outcome, death, or administrative censoring (December 31, 2018).29
Statistical Analysis
Cox proportional hazard models were used to estimate the risk of incident ASCVD events, using separate models for composite ASCVD and each component outcome of ASCVD events (ischemic stroke, MI, or ASCVD death), stratified by HARE group. Sex-stratified models were also estimated for composite ASCVD. Complete case analysis was used for all models, only including patients with nonmissing risk factors. Polygenic scores were validated in ancestry-specific models with standardized PRSs for each outcome, age, sex, and the first 5 principal components of genetic ancestry (G model):
| log[h(t) / h0 (t)] = β1age + β2female + β3-7PCs 1-5 + βTGPRS, |
where h(t) is the hazard for an outcome (composite ASCVD events, stroke, MI, or ASCVD death), h0(t) is the baseline hazard, PCs are principal components, and composite polygenic score (G-score) is βTGPRS = β8PRSAIS + β9PRSCAD for composite ASCVD events and ASCVD death, β8PRSAIS for stroke, and β8PRSCAD for MI. Traditional risk scores were constructed using nongenetic risk factors (E model) from a previously developed VHA model14:
| log[h(t) / h0(t)] = β1Age + β2female + β3diabetes + β4current smoker + β5former smoker + β6-8 total cholesterol(cubic splines) + β9HDL-C + β10SBP + β11BP med Rx. |
Models were estimated in 10-fold cross-validation, with coefficients estimated on training samples, and traditional risk scores (E scores) and composite polygenic scores (G scores) computed for patients in validation folds.
A combined genetic and traditional risk (G × E) model was constructed to evaluate whether polygenic scores improved ASCVD risk prediction over and above traditional risk factors:
| log[hG × E(t) / h0 (t)] = βEE score + βGG score + βG × EE score × G score, |
where E score and G score were combined across the 10 validation folds. This iterative approach to model building is similar to the approach of Steinfeldt et al,30 with their Cox clinical model corresponding to the E model here, Cox Sun PGS model equivalent to the G model, and Cox clinical PGS × age equivalent to our G × E model (just with PRS by age instead of PRS and traditional risk).
Discrimination of the risk models was evaluated using the Harrell C index, with improvement from PRS defined as the difference between genetic and nongenetic models (ΔC = CG × E − CE). Categorical and continuous net reclassification improvement (NRI) were used to assess improvements in risk prediction from addition of PRS.31 To define 5-year risk categories analogous to the guideline-recommended risk categories,32 the primary 10-year risk threshold was halved to define intermediate-risk (3.75% to 10%) and high-risk (>10%) groups. Kaplan-Meier incidence curves were stratified by low (bottom quintile of risk score), moderate (middle 3 quintiles), and high risk (top quintile) using ancestry-specific scores.33 Cox regressions for high- and low-risk groups used a reference of 45th to 55th percentile of risk, adjusting for age, sex, and top 5 principal components of genetic ancestry in PRS models. Data were analyzed from March 15, 2021, to January 5, 2023, using R software, version 4.0.2 (R Foundation for Statistical Computing). Two-sided P values <.05 were considered statistically significant.
Results
The cohort comprised 79 151 veterans (mean [SD] age, 57.8 [13.7] years; 68 503 male [86.5%]; 10 648 female [13.5%]) without baseline ASCVD. As defined by HARE, veterans belonged to the following harmonized genetic ancestry and race and ethnicity categories: 18 505 Black (23.4%), 6785 Hispanic (8.6%), and 53 861 White (68.0%) (Table 1 and eTable 3 in Supplement 1). Black and Hispanic participants were 6 to 10 years younger than White participants on average (mean [SD] age, Black, 53.2 [12.6] years; Hispanic, 49.4 [15.6] years; White, 59.3 [13.8] years). At baseline, 36 673 participants (46.3%) had active prescriptions for antihypertensive therapies, mean (SD) total cholesterol level was 157.8 (40.6) mg/dL (to convert to millimoles per liter, multiply by 0.0259), and prevalence of current smoking was 10.7% (8435), of former smoking was 60.2% (47 670), and of diabetes was 21.7% (17 177) (Table 1).
Table 1. Baseline Characteristics and Atherosclerotic Cardiovascular Disease (ASCVD) Events Among 79 151 Veterans.
| Baseline characteristic | No. (%) | |||
|---|---|---|---|---|
| Total cohort (N = 79 151) | Harmonized ancestry, race, and ethnicity | |||
| Non-Hispanic Black (n = 18 505) | Hispanic (n = 6785) | Non-Hispanic White (n = 53 861) | ||
| Follow-up, median (5th-95th percentile), y | 4.3 (0.7-6.9) | 4.3 (0.7-6.9) | 4.1 (0.6-6.8) | 4.3 (0.6-6.9) |
| Age, mean (SD), y | 57.8 (13.7) | 55.4 (11.8) | 52.6 (14.8) | 59.3 (13.8) |
| Cardiovascular risk factors | ||||
| Sex | ||||
| Male | 68 503 (86.5) | 15 153 (81.9) | 5871 (86.5) | 47 479 (88.2) |
| Female | 10 648 (13.5) | 3352 (18.1) | 914 (13.5) | 6382 (11.8) |
| Total cholesterol, mean (SD), mg/dL | 157.8 (40.6) | 153.7 (40.0) | 156.0 (41.5) | 159.5 (40.6) |
| HDL-C, mean (SD), mg/dL | 49.8 (17.0) | 54.0 (18.1) | 47.4 (15.0) | 48.6 (16.6) |
| LDL-C, mean (SD), mg/dL | 109.4 (33.3) | 106.6 (33.1) | 106.9 (35.0) | 110.6 (33.1) |
| Systolic blood pressure, mean (SD), mm Hg | 131.3 (13.4) | 132.5 (13.7) | 129.6 (13.1) | 131.0 (13.3) |
| Current smoker | 8435 (10.7) | 2034 (11.0) | 812 (12.0) | 5589 (10.4) |
| Former smoker | 47 670 (60.2) | 10 578 (57.2) | 3527 (52.0) | 33 565 (62.3) |
| Diabetes | 17 177 (21.7) | 5173 (28.0) | 1663 (24.5) | 10 341 (19.2) |
| Medications | ||||
| Blood pressure treatment | 36 673 (46.3) | 9390 (50.7) | 2723 (40.1) | 24 560 (45.6) |
| No. of events (2011-2018) | ||||
| Composite CVD (%) | 5485 (6.9) | 1227 (6.6) | 322 (4.7) | 3936 (7.3) |
| Composite CVD and revascularization (%) | 6628 (8.4) | 1428 (7.7) | 411 (6.1) | 4789 (8.9) |
| ASCVD death (%) | 867 (1.1) | 189 (1.0) | 52 (0.8) | 626 (1.2) |
| Acute ischemic stroke (%) | 1933 (2.4) | 481 (2.6) | 124 (1.8) | 1328 (2.5) |
| Myocardial Infarction (%) | 3186 (4.0) | 677 (3.7) | 182 (2.7) | 2327 (4.3) |
| Crude incidence rate per 10 000 person-years (2011-2018) | ||||
| Composite CVD (95% CI) | 177.7 (173.0-182.5) | 168.1 (158.8-177.8) | 124.7 (111.5-139.1) | 187.5 (181.7-193.5) |
| Composite CVD and revascularization (95% CI) | 216.6 (211.4-221.9) | 196.8 (186.7-207.2) | 160.5 (145.4-176.8) | 230.4 (223.9-237.0) |
| ASCVD Death (95% CI) | 27.0 (25.2-28.8) | 24.9 (21.5-28.8) | 19.6 (14.6-25.7) | 28.6 (26.4-30.9) |
| Acute ischemic stroke (95% CI) | 61.0 (58.3-63.8) | 64.4 (58.8-70.4) | 47.3 (39.3-56.4) | 61.5 (58.2-64.9) |
| Myocardial infarction (95% CI) | 100.9 (97.4-104.4) | 90.7 (84.0-97.8) | 69.4 (59.6-80.2) | 108.2 (103.9-112.7) |
Abbreviations: CVD, cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
SI conversion factor: To convert total cholesterol, HDL-C, and LDL-C to millimoles per liter, multiply by 0.0259.
During follow-up (median [5th-95th percentile], 4.3 [0.7-6.9] years), 5485 participants (6.9%) experienced a first ASCVD event, among whom 3186 (4.0%) experienced an MI, 1933 (2.4%) experienced an ischemic stroke, and 867 (1.1%) died of an ASCVD event, including multiple possible events for each individual. Women had fewer first ASCVD events than men, with only 310 composite ASCVD cases (2.9%) compared with 5175 (7.6%), respectively. Crude incidence rate of composite ASCVD events was greatest among HARE-defined White participants (187.5; 95% CI, 181.7-193.5 events per 10 000 person-years [PY]), slightly lower for Black participants (168.1; 95% CI, 158.8-177.8 events per 10 000 PY), and much lower for Hispanic participants (124.7; 95% CI, 111.5-139.1 events per 10 000 PY) (Table 1).
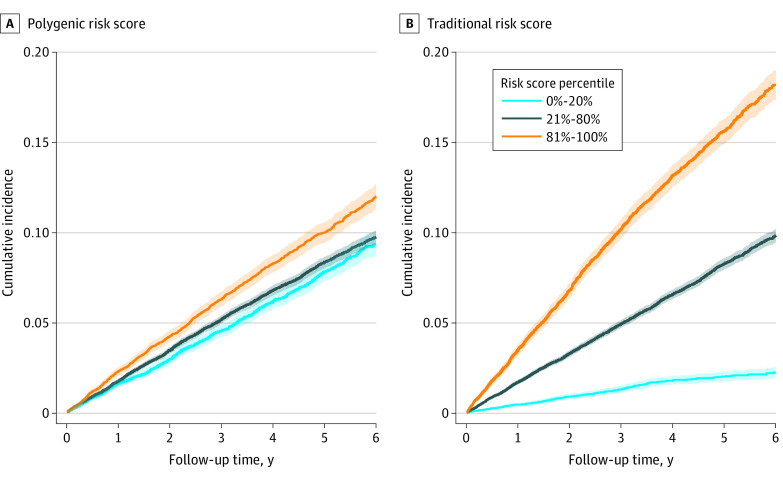
Crude cumulative incidence curves for composite ASCVD events are presented in Figure 1 and for each ASCVD outcome in eFigure 1 in Supplement 1, stratified by percentile of composite PRS and traditional risk score. Both scores generally described a gradient of risk within the component and composite outcomes. The magnitude of this gradient from the lowest to highest percentiles was greater for the traditional risk score than the PRS. The trend in cumulative incidence of composite ASCVD events across PRS was largely driven by the CAD PRS.
Figure 1. Cumulative Incidence of Composite Atherosclerotic Cardiovascular Disease Events.
Cumulative incidence curves for low (light blue line), moderate (dark blue line), and high (orange line) predicted polygenic (A) and traditional (B) risk are presented. Shaded regions are 95% exact Poisson CIs.
Adjusted (for age, sex, and principal components of genetic ancestry) hazard ratios (HRs) for more extreme risk groups up to the top 0.5 percentile of PRS and traditional risk score are presented in Table 2 and down to the bottom 0.5 percentile in eTable 4 in Supplement 1, compared with the middle decile of risk (45%-55%). The HRs differed by HARE group: a 1-SD increase of composite PRS increased the hazard for composite ASCVD events by 11% (95% CI, 5%-17%) in Black veterans, 12% (95% CI, 0-25%) in Hispanic veterans, and 20% (95% CI, 16%-24%) in White veterans. The per-SD HRs for traditional ASCVD risk scores were an order of magnitude greater than PRS in Black participants (HR, 1.96; 95% CI, 1.84-2.09), Hispanic participants (HR, 2.29; 95% CI, 2.02-2.59), and White participants (HR, 1.93; 95% CI, 1.86-2.00). In analyses of PRS categories, among White participants, the risk for composite ASCVD was positively associated with higher PRS groups, ranging from an HR of 1.42 (95% CI, 1.26-1.61) in the top 20% (compared with the middle decile) to an HR of 2.21 (95% CI, 1.55-3.15) in the top 0.5% of PRS, which was comparable with the top 0.5% of traditional risk score (HR, 2.62; 95% CI, 1.95-3.52).
Table 2. Incident Cardiovascular Disease (CVD) Hazard Ratios for High Traditional and Polygenic Risk Scores (PRSs).
| High PRS definition | Hazard ratio (95% CI) | |||||
|---|---|---|---|---|---|---|
| Non-Hispanic Black | Hispanic | Non-Hispanic White | ||||
| PRS | Traditional risk score | PRS | Traditional risk score | PRS | Traditional risk score | |
| Composite CVD (CAD PRS + AIS PRS) | ||||||
| Continuous per SD increment | 1.11 (1.05-1.17) | 1.96 (1.84-2.09) | 1.12 (1.00-1.25) | 2.29 (2.02-2.59) | 1.20 (1.16-1.24) | 1.93 (1.86-2.00) |
| Top 20%a | 1.18 (0.95-1.46) | 1.94 (1.60-2.36) | 0.79 (0.54-1.16) | 3.07 (2.03-4.67) | 1.42 (1.26-1.61) | 1.76 (1.58-1.96) |
| Top 10% | 1.29 (1.02-1.64) | 2.01 (1.62-2.50) | 0.83 (0.53-1.32) | 3.24 (2.07-5.07) | 1.56 (1.36-1.79) | 2.08 (1.85-2.34) |
| Top 5% | 1.20 (0.90-1.61) | 2.30 (1.81-2.93) | NA | 3.93 (2.40-6.41) | 1.53 (1.30-1.81) | 2.40 (2.10-2.74) |
| Top 1% | NAb | 3.07 (2.10-4.48) | NA | NA | 2.13 (1.63-2.79) | 2.69 (2.17-3.34) |
| Top 0.5% | NA | NA | NA | NA | 2.21 (1.55-3.15) | 2.62 (1.95-3.52) |
| Myocardial infarction (CAD PRS) | ||||||
| Continuous per SD increment | 1.10 (1.02-1.19) | 2.01 (1.83-2.20) | 1.26 (1.09-1.46) | 2.46 (2.07-2.92) | 1.23 (1.18-1.29) | 1.97 (1.87-2.06) |
| Top 20% | 1.25 (0.93-1.68) | 2.17 (1.64-2.87) | 0.71 (0.45-1.13) | 3.60 (1.96-6.59) | 1.36 (1.16-1.58) | 2.05 (1.77-2.37) |
| Top 10% | 1.35 (0.97-1.88) | 2.32 (1.71-3.15) | NA | 4.09 (2.17-7.74) | 1.50 (1.26-1.78) | 2.32 (1.98-2.71) |
| Top 5% | 1.40 (0.95-2.05) | 2.82 (2.02-3.95) | NA | 5.47 (2.80-10.69) | 1.61 (1.31-1.96) | 2.63 (2.20-3.13) |
| Top 1% | NA | 4.06 (2.49-6.62) | NA | NA | 2.23 (1.61-3.08) | 3.61 (2.77-4.70) |
| Top 0.5% | NA | NA | NA | NA | 2.19 (1.40-3.43) | 3.09 (2.12-4.49) |
| Ischemic stroke (AIS PRS) | ||||||
| Continuous per SD increment | 1.05 (0.95-1.17) | 1.90 (1.73-2.08) | 1.08 (0.85-1.36) | 2.09 (1.73-2.52) | 1.15 (1.08-1.21) | 1.79 (1.68-1.90) |
| Top 20% | 0.85 (0.60-1.19) | 2.48 (1.76-3.51) | 1.71 (0.74-3.94) | 2.14 (1.19-3.84) | 1.22 (0.99-1.50) | 1.64 (1.36-1.98) |
| Top 10% | 0.94 (0.63-1.41) | 2.77 (1.92-4.01) | NA | 2.55 (1.36-4.78) | 1.21 (0.95-1.53) | 2.01 (1.64-2.46) |
| Top 5% | 1.29 (0.82-2.04) | 2.90 (1.92-4.38) | NA | NA | 1.16 (0.86-1.56) | 2.43 (1.95-3.04) |
| Top 1% | NA | NA | NA | NA | 1.82 (1.14-2.90) | 3.06 (2.16-4.35) |
| Top 0.5% | NA | NA | NA | NA | NA | NA |
| ASCVD death (CAD PRS + AIS PRS) | ||||||
| Continuous per SD increment | 1.19 (1.03-1.37) | 2.61 (2.20-3.10) | 1.24 (0.94-1.65) | 3.84 (2.72-5.41) | 1.11 (1.03-1.21) | 2.60 (2.34-2.88) |
| Top 20% | 1.30 (0.77-2.19) | 2.89 (1.69-4.93) | NA | 8.06 (1.93-33.68) | 1.32 (0.97-1.81) | 2.39 (1.81-3.15) |
| Top 10% | 1.36 (0.76-2.45) | 3.42 (1.95-6.01) | NA | NA | 1.60 (1.13-2.27) | 3.42 (2.58-4.55) |
| Top 5% | NA | 4.54 (2.50-8.22) | NA | NA | 1.49 (0.97-2.28) | 3.92 (2.88-5.33) |
| Top 1% | NA | NA | NA | NA | NA | 5.41 (3.52-8.33) |
| Top 0.5% | NA | NA | NA | NA | NA | NA |
Abbreviations: AIS, acute ischemic stroke; ASCVD, atherosclerotic cardiovascular disease; CAD, coronary artery disease; NA, not applicable.
Reference group equals middle 10% (45th to 55th percentiles) of risk score.
NA, less than 10 events in risk group.
Among component outcomes of ASCVD events, CAD PRS was associated with incident MI in non-Hispanic Black (HR, 1.10; 95% CI, 1.02-1.19), Hispanic (HR, 1.26; 95% CI, 1.09-1.46), and non-Hispanic White (HR, 1.23; 95% CI, 1.18-1.29) participants. Stroke PRS was associated with incident stroke in non-Hispanic White participants (HR, 1.15; 95% CI, 1.08-1.21). The combined CAD plus stroke PRS was associated with ASCVD deaths among non-Hispanic Black (HR, 1.19; 95% CI, 1.03-1.17) and non-Hispanic White (HR, 1.11; 95% CI, 1.03-1.21) participants.
The Harrell C indices for each incident ASCVD outcome are presented in Figure 2 for the traditional risk model, combined traditional and polygenic risk model, and improvement of combined model over traditional risk model. The C statistics for traditional risk score (E model) and G × E models ranged from 0.64 to 0.68 for ASCVD outcomes (composite ASCVD events, MI, and stroke) and 0.70 to 0.71 for ASCVD death among HARE-defined non-Hispanic Black and White participants. Concordance of the traditional and G × E model was greater for Hispanic veterans, ranging from 0.70 to 0.73 for all outcomes except ASCVD death (C index, 0.78; 95% CI, 0.75-0.80). The addition of PRS to the traditional model (G × E vs E) increased the C index for all ASCVD outcomes by roughly 0.01 among Hispanic and White participants, but the incremental effects were smaller (C index, 0.004; 95% CI, 0-0.011) for ischemic stroke and for all nondeath outcomes among Black participants (composite CVD: C index, 0.004; 95% CI, 0.002-0.006; MI: C index, 0.006; 95% CI, 0.002-0.010).
Figure 2. Harrell C Index for Traditional (Model E) and Combined Gene-Environment (Model G × E) Risk Models.
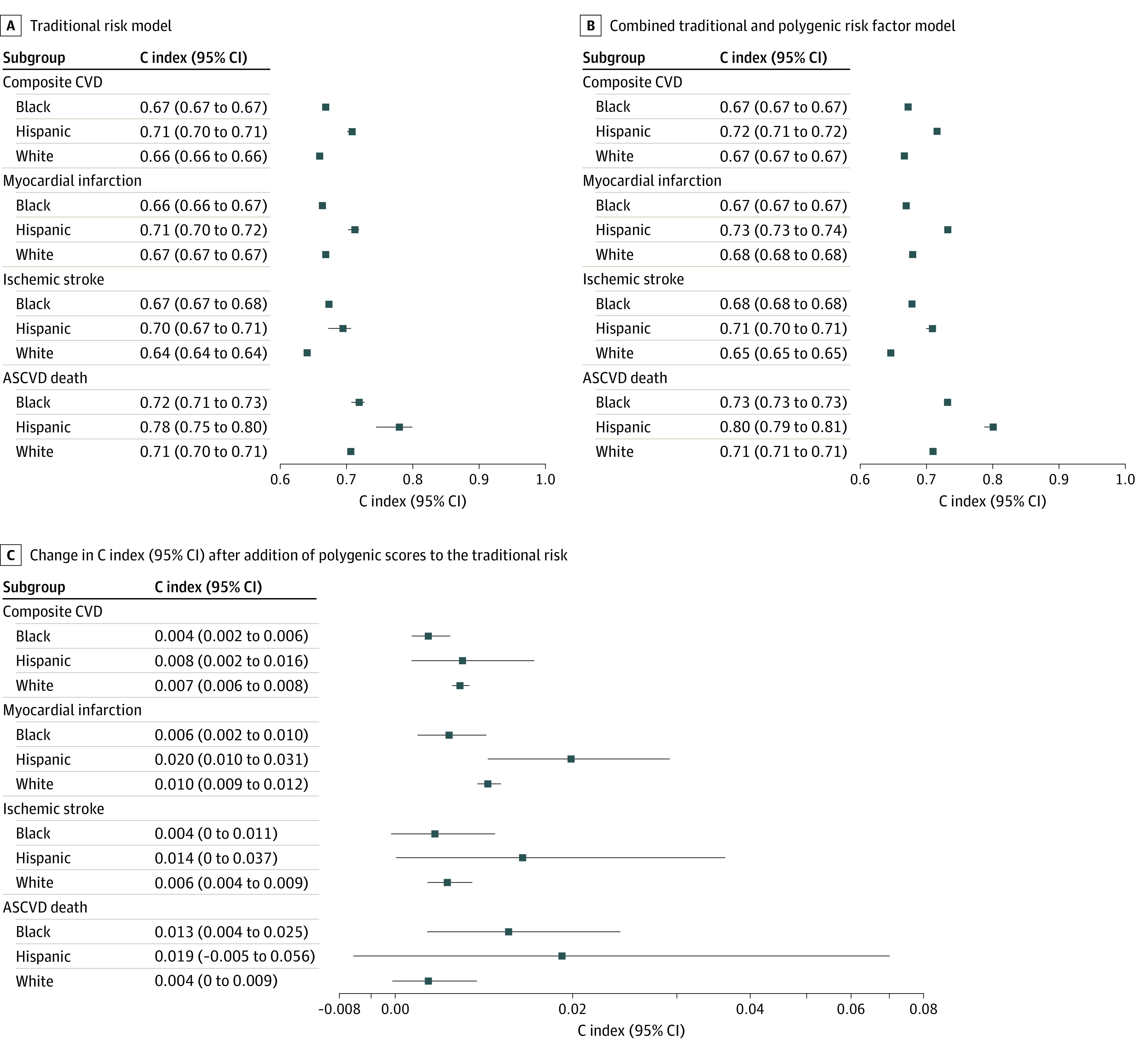
Harrell C index of the ancestry-specific risk models for each atherosclerotic cardiovascular disease (ASCVD) outcome is presented. The line segments represent 95% CIs constructed from 1000 bootstrap samples. A, Traditional risk model. B, Combined traditional and polygenic risk factor (G × E) model including an interaction between traditional and polygenic risk scores. C, Improvement in C index from the addition of polygenic scores to the traditional risk. C index ranges from 0.5 (which indicates a risk score is no better than a coin flip at predicting which patient [in each pair of patients] will have an event first) to 1, which indicates a risk score perfectly predicts which patient (of each pair) has an event first. Change in C index is calculated by subtracting the traditional model’s C index from the G × E model’s C index, with positive values indicating an improvement from incorporation of polygenic scores. CVD indicates cardiovascular disease.
Adding PRS to the traditional ASCVD risk model significantly improved reclassification of women around the intermediate risk threshold (5-year risk >3.5%) by 6.8% (95% CI, 3.0%-10.6%) across all HARE groups combined (eTable 8 in Supplement 1), with the most reclassification occurring among Hispanic women at 19.0% (95% CI, 2.1%-39.9%), followed by Black women at 10.6% (95% CI, 3.1%-18.4%), and lowest among White women at 3.4% (95% CI, −0.1% to 7.4%) (Figure 3A). NRI of men was modest for the intermediate risk group for composite CVD (5-year risk >3.75%, 0.38%; 95% CI, 0.07%-0.68%) and significant around the high-risk threshold (5-year risk >10%) at 1.9% (95% CI, 1.0%-2.8%) overall, 1.9% (95% CI, 1.0%-3.0%) among White veterans (Figure 3B), and 3.2% (95% CI, −0.1% to 6.4%) among Hispanic veterans. Across both risk thresholds, NRI was approximately 1.4% higher in middle-aged adults aged 40 to 55 years (1.61%; 95% CI, −0.07% to 3.30%) compared with participants older than 55 years (0.25%; 95% CI, 0.03%-0.47%), with a net 3.2% (95% CI, 1.4%-5.0%) of middle-aged reclassified above or below high risk compared with the 1.8% (95% CI, 1.4%-5.0%) among participants older than 55 years. NRI was not present among Black participants except around the intermediate risk threshold for women. Hispanic participants had higher reclassification rates compared with White participants across all subgroups except for the intermediate risk group of middle-aged adults.
Figure 3. Net Reclassification Improvement for Composite Cardiovascular Disease (CVD) From Inclusion of Polygenic Risk Scores.
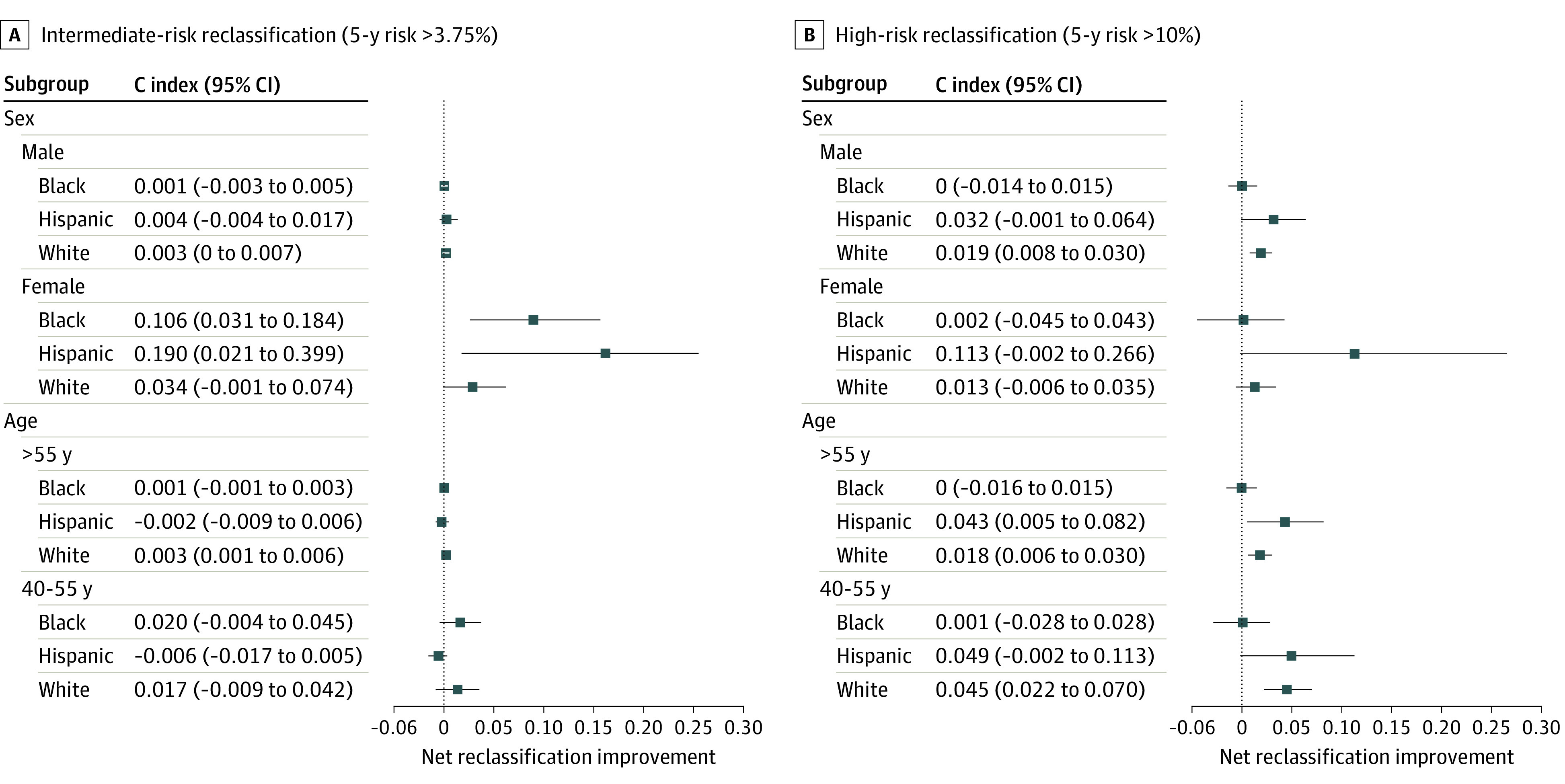
A and B present net intermediate and high-risk reclassification improvement, respectively, from the addition of polygenic scores to the traditional risk model for composite CVD, stratified by ancestry, age group, and sex.
Although there were no reclassifications of the youngest statin-naive participants younger than 40 years (eFigure 2 in Supplement 1), a cohort including statin users exhibited a decreasing trend in reclassification with age: greatest among young participants, reduced in middle-aged adults, and lowest for older adults (eFigure 3 in Supplement 1). The median age of first ASCVD event was also 10 years lower in the top decile of PRS compared with traditional risk score (eFigure 4 in Supplement 1).
Continuous NRI (eFigure 5 in Supplement 1) was weak (<0.2) for HARE-defined Black and White participants and moderate for Hispanic participants (0.2 to 0.4) across all ASCVD outcomes, except ASCVD death. Compared with the traditional risk model, the combined traditional and PRS model predicted a greater ASCVD risk among events (and reduced risk for nonevents) for a net 12.2% (95% CI, 5.8%-18.4%) of Black participants, 26.0% (95% CI, 13.6%-37.1%) of Hispanic participants, and 13.8% (95% CI, 10.0%-17.4%) of White participants. Continuous NRI increased when including statin users and was moderate across all HARE groups at 14.4% (95% CI, 8.1%-20.5%) among Black participants, 25.7% (95% CI, 14.7%-36.3%) among Hispanic participants, and 20.1% (95% CI, 16.7%-23.7%) among White participants. Reclassification tables for other ASCVD outcomes are presented in eTables 5 to 7 in Supplement 1.
Discussion
In this large multiancestry prognostic cohort study, PRSs derived from both CAD and ischemic stroke GWAS summary statistics were associated with incident ASCVD events in a primary prevention population. Among the 3 populations examined, PRSs were statistically significantly associated with incident MI, ischemic stroke, ASCVD death, and composite ASCVD events with a higher (but still modest) degree of improved reclassification observed for women compared with men and for younger-onset disease compared with older-onset disease.
Previous studies of incident ASCVD in predominantly European-ancestry populations have reported small but consistent improvements in model performance when adding PRS.34 These studies have demonstrated changes in C statistics between 0.009 to 0.02 and 10-year NRI between 2.7% to 4.7%. Fewer studies have evaluated PRS in non-European cohorts and have generally observed attenuated magnitudes of association and reclassification.4,5,12 The present study included 18 505 Black and 6785 Hispanic participants, representing an important contribution to polygenic ASCVD risk stratification research, where to date the largest study has included only 55 ASCVD events among 823 Hispanic participants.4 Similar to prior reports, European-derived PRS was significantly associated with incident ASCVD events in Black and Hispanic MVP participants but with reduced or no improvement in net reclassification. Continued advances in multiancestry GWAS and PRS development are expected to further improve PRS performance in populations of diverse genetic ancestry.35 Whether to include the sociocultural constructs of race and ethnicity in clinical prediction models is controversial but remains the standard of care in ASCVD risk estimation and prevention.36,37 Further research is needed before consensus can be reached about the role of socially defined race and ethnicity and genetically defined ancestry in risk prediction, with or without PRS.38,39
Although most other studies have evaluated a CAD PRS for ASCVD risk stratification, this study examined the utility of published PRSs for 2 components of ASCVD (CAD and ischemic stroke) across 4 ASCVD outcomes (MI, ischemic stroke, ASCVD death, and composite ASCVD events). Physicians use models that estimate 10-year risk of ASCVD events, and thus, the inclusion of both CAD- and stroke-associated PRS may be more appropriate to evaluate the potential for PRSs to improve existing clinical models. Across HARE groups, this study observed that the CAD PRS was associated with incident MI, the stroke PRS was associated with incident ischemic stroke, and the combination of the 2 PRSs was associated with ASCVD death and composite ASCVD events. In all models, the risk scores constructed from traditional risk factors conferred greater ASCVD risk per SD compared with PRS. Further research is needed to determine the best approach to combining PRS for clinical risk stratification of multiple phenotypes, perhaps leveraging transcriptomic data or more advanced computational approaches to PRS construction.4,30,40 Improved understanding of the genetic architecture of CVD may inform a paradigm change in clinical practice, favoring the identification and risk management of more precise disease phenotypes and corresponding prevention strategies. Either way, for a clinical prediction model to be widely implemented, it requires inputs that are already clinically available or readily attainable, and several challenges impede the implementation of increasingly complex prediction models at the point of patient care.
Despite few cases and low statistical power among younger strata, the higher NRI observed among younger participants in the MVP is largely concordant with independent studies of Europeans aged 40 to 55 years (NRI = 10.3%)4 and younger than 50 years (13.5% reclassified to intermediate risk) in the UK41 and Black participants in the US (8.5%). A Finnish study similarly observed that 12.6% of the coronary events that occurred before 55 years of age were attributable to high PRS risk compared with 2.5% for later-onset events.42 The older VHA patient population and analysis of first ASCVD events after MVP enrollment likely excluded other early-life events where genetic factors may contribute more to ASCVD development. Still, to our knowledge, this was the first multiancestry study to evaluate PRS for predicting first ASCVD events in individuals younger than 40 years.43,44
Limitations
There are a few study limitations to note. First, the PRSs used were developed in predominantly European ancestry populations and were not multiancestry or transancestry scores. Second, due to limited follow-up in the MVP, only 5-year ASCVD risk was estimated instead of the usual 10-year risk. Third, the high rate of statin use at baseline and the large number of participants with clinical CAD who may have undergone revascularization procedures without experiencing a qualifying outcome during the short follow-up period may have reduced the discriminative potential of a PRS for CAD across all ages. Supporting this possibility is a growing body of evidence demonstrating that participants in the highest tertile of a PRS derive the greatest benefit from a statin, with a near halving of risk in both primary and secondary prevention settings, whereas those in the bottom tertile derive little to no benefit over the same period of follow-up.45,46,47 Lastly, demographic composition was predominantly male. Although the sample included almost 11 000 women, sex-specific differences in event rates may make the results less generalizable to women.
Whether the improved discrimination observed in this study (and others) supports the integration of PRSs in clinical practice cannot be answered definitively without clinical trials.48,49 Arguments against clinical utility of PRSs have historically been rooted in the low absolute magnitude of incremental discrimination such as the delta C statistic—a low-power procedure—or the NRI.48,50 However, there remains considerable debate on which metrics should be used to demonstrate clinical validity. Net benefit, for example, may be most appropriate when the harm of overtreatment is minimal, such as lifestyle counseling.31,51,52,53,54,55,56 The 2019 American College of Cardiology/American Heart Association cholesterol guideline emphasizes shared decision-making between physician and patients at moderate or high 10-year risk (ie, >5.0%), including consideration of risk-enhancing factors beyond traditional ASCVD risk models.57 Under these circumstances, a specific threshold of risk does not exist and even small improvements in discrimination may be clinically helpful when applied to large populations at risk. The recent American Heart Association scientific statement on PRSs further clarifies these potential benefits for CAD risk stratification.11 It may be reasonable to consider a PRS as an additional risk-enhancing factor based on the performance of the most recently constructed genome-wide PRS. This may be better supported if anticipated improvements in PRSs are actualized over time.49
Conclusions
In conclusion, results of this prognostic study suggest that PRSs for CAD and ischemic stroke derived from largely European ancestry populations were statistically significantly associated with first ASCVD events in the multiancestry MVP cohort, but reclassification improvement was modest. This study also reinforces the need for multiancestry PRSs to improve risk stratification of non-European populations. The results suggest the possibility that the predictive utility of PRS may be relatively higher for women and younger populations, but more studies, especially clinical trials, are needed before definitive conclusions can be made in this regard.
eTable 1. Genetically Informed Ancestry (GIA) and HARE Classification in the Million Veteran Program (MVP)
eTable 2. Electronic Health Record Codes (ICD-9, ICD-10, CPT/HCPCS) for ASCVD Outcomes
eTable 3. Baseline Characteristics and ASCVD Events Stratified by Sex
eTable 4. Hazard Ratios for MVP Incident ASCVD Events for Low Traditional and Polygenic Risk Scores
eTable 5. Reclassification of 5-Year Predicted Myocardial Infarction Including Both Statin-Naïve and Statin Users
eTable 6. Reclassification of 5-Year Predicted Acute Ischemic Stroke Including Both Statin-Naïve and Statin Users
eTable 7. Reclassification of 5-Year Predicted ASCVD Death Including Both Statin-Naïve and Statin Users
eTable 8. Net Reclassification Improvement From Inclusion of Polygenic Scores Stratified by Age and Sex
eFigure 1. Cumulative Incidence of ASCVD Events
eFigure 2. Categorical Net Reclassification Index for Incident Composite ASCVD Among Statin-Naïve Participants Outcomes Stratified by Age Group
eFigure 3. Net Reclassification Index for Intermediate Risk (5-Year Risk >3.75%) From Inclusion of Polygenic Risk Scores Including Statin Users Stratified by Age
eFigure 4. Age of Onset for Incident ASCVD
eFigure 5. Continuous and Categorical Net Reclassification Index for Incident ASCVD Outcomes
Data Sharing Statement
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153-e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 2.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129(25)(suppl 2):S49-S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 3.Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease pooled cohort risk equations. JAMA. 2014;311(14):1406-1415. doi: 10.1001/jama.2014.2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weale ME, Riveros-Mckay F, Selzam S, et al. Validation of an integrated risk tool, including polygenic risk score, for atherosclerotic cardiovascular disease in multiple ethnicities and ancestries. Am J Cardiol. 2021;148:157-164. doi: 10.1016/j.amjcard.2021.02.032 [DOI] [PubMed] [Google Scholar]
- 5.Dikilitas O, Schaid DJ, Kosel ML, et al. Predictive utility of polygenic risk scores for coronary heart disease in 3 major racial and ethnic groups. Am J Hum Genet. 2020;106(5):707-716. doi: 10.1016/j.ajhg.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muse ED, Chen SF, Liu S, et al. Impact of polygenic risk communication: an observational mobile application-based coronary artery disease study. NPJ Digit Med. 2022;5(1):30. doi: 10.1038/s41746-022-00578-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao L, Kraft P, Berriz GF, et al. Development of a clinical polygenic risk score assay and reporting workflow. Nat Med. 2022;28(5):1006-1013. doi: 10.1038/s41591-022-01767-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Human Genome Research Institute . eMERGE Network. Accessed July 14, 2022. https://www.genome.gov/Funded-Programs-Projects/Electronic-Medical-Records-and-Genomics-Network-eMERGE
- 9.Khera AV, Chaffin M, Aragam KG, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018;50(9):1219-1224. doi: 10.1038/s41588-018-0183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun L, Pennells L, Kaptoge S, et al. Polygenic risk scores in cardiovascular risk prediction: a cohort study and modelling analyses. PLoS Med. 2021;18(1):e1003498. doi: 10.1371/journal.pmed.1003498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Sullivan JW, Raghavan S, Marquez-Luna C, et al. ; American Heart Association Council on Genomic and Precision Medicine; Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease . Polygenic risk scores for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2022;146(8):e93-e118. doi: 10.1161/CIR.0000000000001077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosley JD, Gupta DK, Tan J, et al. Predictive accuracy of a polygenic risk score compared with a clinical risk score for incident coronary heart disease. JAMA. 2020;323(7):627-635. doi: 10.1001/jama.2019.21782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaziano JM, Concato J, Brophy M, et al. Million Veteran Program: a mega-biobank to study genetic influences on health and disease. J Clin Epidemiol. 2016;70:214-223. doi: 10.1016/j.jclinepi.2015.09.016 [DOI] [PubMed] [Google Scholar]
- 14.Vassy JL, Lu B, Ho YL, et al. Estimation of atherosclerotic cardiovascular disease risk among patients in the Veterans Affairs health care system. JAMA Netw Open. 2020;3(7):e208236. doi: 10.1001/jamanetworkopen.2020.8236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang H, Hui Q, Lynch J, et al. ; VA Million Veteran Program . Harmonizing genetic ancestry and self-identified race/ethnicity in genome-wide association studies. Am J Hum Genet. 2019;105(4):763-772. doi: 10.1016/j.ajhg.2019.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryc K, Velez C, Karafet T, et al. Colloquium paper: genome-wide patterns of population structure and admixture among Hispanic/Latino populations. Proc Natl Acad Sci U S A. 2010;107(suppl 2):8954-8961. doi: 10.1073/pnas.0914618107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price LE, Shea K, Gephart S. The Veterans Affairs’s corporate data warehouse: uses and implications for nursing research and practice. Nurs Adm Q. 2015;39(4):311-318. doi: 10.1097/NAQ.0000000000000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Department of Veterans Affairs . Veteran suicide surveillance: methods summary. Accessed March 26, 2023. https://www.mentalhealth.va.gov/docs/data-sheets/2022/2022-National-Veteran-Suicide-Prevention-Annual-Report-Methods-Summary-FINAL-508.pdf
- 19.US Department of Veterans Affairs . VA Privacy Act system of records. Accessed March 26, 2023. https://www.oprm.va.gov/docs/SORN/Current_SORN_List_02_28_2023.pdf
- 20.Hunter-Zinck H, Shi Y, Li M, et al. ; VA Million Veteran Program . Genotyping array design and data quality control in the Million Veteran Program. Am J Hum Genet. 2020;106(4):535-548. doi: 10.1016/j.ajhg.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra A, Malik R, Hachiya T, et al. ; COMPASS Consortium; INVENT Consortium; Dutch Parelsnoer Initiative (PSI) Cerebrovascular Disease Study Group; Estonian Biobank; PRECISE4Q Consortium; FinnGen Consortium; NINDS Stroke Genetics Network (SiGN); MEGASTROKE Consortium; SIREN Consortium; China Kadoorie Biobank Collaborative Group; VA Million Veteran Program; International Stroke Genetics Consortium (ISGC); Biobank Japan; CHARGE Consortium; GIGASTROKE Consortium . Stroke genetics informs drug discovery and risk prediction across ancestries. Nature. 2022;611(7934):115-123. doi: 10.1038/s41586-022-05165-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mars N, Kerminen S, Feng YA, et al. Genome-wide risk prediction of common diseases across ancestries in 1 million people. Cell Genom. 2022;2(4):None. doi: 10.1016/j.xgen.2022.100118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert SA, Gil L, Jupp S, et al. The polygenic score catalog as an open database for reproducibility and systematic evaluation. Nat Genet. 2021;53(4):420-425. doi: 10.1038/s41588-021-00783-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auton A, Brooks LD, Durbin RM, et al. ; 1000 Genomes Project Consortium . A global reference for human genetic variation. Nature. 2015;526(7571):68-74. doi: 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurdasani D, Carstensen T, Tekola-Ayele F, et al. The African Genome Variation Project shapes medical genetics in Africa. Nature. 2015;517(7534):327-332. doi: 10.1038/nature13997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song RJ, Ho YL, Nguyen XMT, et al. ; Million Veteran Program Data Analytics Group . Development of an electronic health record–based algorithm for smoking status using the Million Veteran Program (MVP) cohort survey response. Circulation. 2016;134(1):A18809. [Google Scholar]
- 27.Imran TF, Posner D, Honerlaw J, et al. A phenotyping algorithm to identify acute ischemic stroke accurately from a national biobank: the Million Veteran Program. Clin Epidemiol. 2018;10:1509-1521. doi: 10.2147/CLEP.S160764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Cai T, Yu S, et al. High-throughput phenotyping with electronic medical record data using a common semi-supervised approach (PheCAP). Nat Protoc. 2019;14(12):3426-3444. doi: 10.1038/s41596-019-0227-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke. 2002;33(10):2465-2470. doi: 10.1161/01.STR.0000032240.28636.BD [DOI] [PubMed] [Google Scholar]
- 30.Steinfeldt J, Buergel T, Loock L, et al. Neural network-based integration of polygenic and clinical information: development and validation of a prediction model for 10-year risk of major adverse cardiac events in the UK Biobank cohort. Lancet Digit Health. 2022;4(2):e84-e94. doi: 10.1016/S2589-7500(21)00249-1 [DOI] [PubMed] [Google Scholar]
- 31.Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157-172. doi: 10.1002/sim.2929 [DOI] [PubMed] [Google Scholar]
- 32.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;140(11):e596-e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khera AV, Emdin CA, Drake I, et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N Engl J Med. 2016;375(24):2349-2358. doi: 10.1056/NEJMoa1605086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan SS, Page C, Wojdyla DM, Schwartz YY, Greenland P, Pencina MJ. Predictive utility of a validated polygenic risk score for long-term risk of coronary heart disease in young and middle-aged adults. Circulation. 2022;146(8):587-596. doi: 10.1161/CIRCULATIONAHA.121.058426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Human genome Research Institute . Polygenic risk methods in diverse populations (PRIMED) consortium. Accessed March 26, 2023. https://www.genome.gov/Funded-Programs-Projects/PRIMED-Consortium
- 36.Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight—reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383(9):874-882. doi: 10.1056/NEJMms2004740 [DOI] [PubMed] [Google Scholar]
- 37.Vasan RS, van den Heuvel E. Differences in estimates for 10-year risk of cardiovascular disease in Black vs White individuals with identical risk factor profiles using pooled cohort equations: an in silico cohort study. Lancet Digit Health. 2022;4(1):e55-e63. doi: 10.1016/S2589-7500(21)00236-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carnethon MR, Pu J, Howard G, et al. ; American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council . Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136(21):e393-e423. doi: 10.1161/CIR.0000000000000534 [DOI] [PubMed] [Google Scholar]
- 39.Lewis ACF, Molina SJ, Appelbaum PS, et al. Getting genetic ancestry right for science and society. Science. 2022;376(6590):250-252. doi: 10.1126/science.abm7530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu X, Qiao D, Kim W, et al. ; TOPMed Lung Working Group . Polygenic transcriptome risk scores for COPD and lung function improve cross-ethnic portability of prediction in the NHLBI TOPMed program. Am J Hum Genet. 2022;109(5):857-870. doi: 10.1016/j.ajhg.2022.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marston NA, Pirruccello JP, Melloni GEM, et al. Predictive utility of a coronary artery disease polygenic risk score in primary prevention. JAMA Cardiol. 2023;8(2):130-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mars N, Koskela JT, Ripatti P, et al. ; FinnGen . Polygenic and clinical risk scores and their impact on age at onset and prediction of cardiometabolic diseases and common cancers. Nat Med. 2020;26(4):549-557. doi: 10.1038/s41591-020-0800-0 [DOI] [PubMed] [Google Scholar]
- 43.Stone NJ, Smith SC Jr, Orringer CE, et al. Managing atherosclerotic cardiovascular risk in young adults: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(8):819-836. doi: 10.1016/j.jacc.2021.12.016 [DOI] [PubMed] [Google Scholar]
- 44.Emdin CA, Xia R, Agrawal S, et al. Polygenic score assessed in young adulthood and onset of subclinical atherosclerosis and coronary heart disease. J Am Coll Cardiol. 2022;80(3):280-282. doi: 10.1016/j.jacc.2022.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mega JL, Stitziel NO, Smith JG, et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet. 2015;385(9984):2264-2271. doi: 10.1016/S0140-6736(14)61730-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Natarajan P, Young R, Stitziel NO, et al. Polygenic risk score identifies subgroup with higher burden of atherosclerosis and greater relative benefit from statin therapy in the primary prevention setting. Circulation. 2017;135(22):2091-2101. doi: 10.1161/CIRCULATIONAHA.116.024436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oni-Orisan A, Haldar T, Cayabyab MAS, et al. Polygenic risk score and statin relative risk reduction for primary prevention of myocardial infarction in a real-world population. Clin Pharmacol Ther. 2022;112(5):1070-1078. doi: 10.1002/cpt.2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumuthini J, Zick B, Balasopoulou A, et al. ; G2MC Evidence investigators . The clinical utility of polygenic risk scores in genomic medicine practices: a systematic review. Hum Genet. 2022;141(11):1697-1704. doi: 10.1007/s00439-022-02452-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klarin D, Natarajan P. Clinical utility of polygenic risk scores for coronary artery disease. Nat Rev Cardiol. 2022;19(5):291-301. doi: 10.1038/s41569-021-00638-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang L, Chen W, Petrick NA, Gallas BD. Comparing 2 correlated C indices with right-censored survival outcome: a one-shot nonparametric approach. Stat Med. 2015;34(4):685-703. doi: 10.1002/sim.6370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115(7):928-935. doi: 10.1161/CIRCULATIONAHA.106.672402 [DOI] [PubMed] [Google Scholar]
- 52.Pepe MS, Kerr KF, Longton G, Wang Z. Testing for improvement in prediction model performance. Stat Med. 2013;32(9):1467-1482. doi: 10.1002/sim.5727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pepe MS, Janes H, Li CI. Net risk reclassification P values: valid or misleading? J Natl Cancer Inst. 2014;106(4):dju041. doi: 10.1093/jnci/dju041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leening MJ, Vedder MM, Witteman JC, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician’s guide. Ann Intern Med. 2014;160(2):122-131. doi: 10.7326/M13-1522 [DOI] [PubMed] [Google Scholar]
- 55.Janes H, Pepe MS, McShane LM, Sargent DJ, Heagerty PJ. The fundamental difficulty with evaluating the accuracy of biomarkers for guiding treatment. J Natl Cancer Inst. 2015;107(8):djv157. doi: 10.1093/jnci/djv157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vickers AJ, van Calster B, Steyerberg EW. A simple, step-by-step guide to interpreting decision curve analysis. Diagn Progn Res. 2019;3:18. doi: 10.1186/s41512-019-0064-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;74(10):e177-e232. doi: 10.1016/j.jacc.2019.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Genetically Informed Ancestry (GIA) and HARE Classification in the Million Veteran Program (MVP)
eTable 2. Electronic Health Record Codes (ICD-9, ICD-10, CPT/HCPCS) for ASCVD Outcomes
eTable 3. Baseline Characteristics and ASCVD Events Stratified by Sex
eTable 4. Hazard Ratios for MVP Incident ASCVD Events for Low Traditional and Polygenic Risk Scores
eTable 5. Reclassification of 5-Year Predicted Myocardial Infarction Including Both Statin-Naïve and Statin Users
eTable 6. Reclassification of 5-Year Predicted Acute Ischemic Stroke Including Both Statin-Naïve and Statin Users
eTable 7. Reclassification of 5-Year Predicted ASCVD Death Including Both Statin-Naïve and Statin Users
eTable 8. Net Reclassification Improvement From Inclusion of Polygenic Scores Stratified by Age and Sex
eFigure 1. Cumulative Incidence of ASCVD Events
eFigure 2. Categorical Net Reclassification Index for Incident Composite ASCVD Among Statin-Naïve Participants Outcomes Stratified by Age Group
eFigure 3. Net Reclassification Index for Intermediate Risk (5-Year Risk >3.75%) From Inclusion of Polygenic Risk Scores Including Statin Users Stratified by Age
eFigure 4. Age of Onset for Incident ASCVD
eFigure 5. Continuous and Categorical Net Reclassification Index for Incident ASCVD Outcomes
Data Sharing Statement