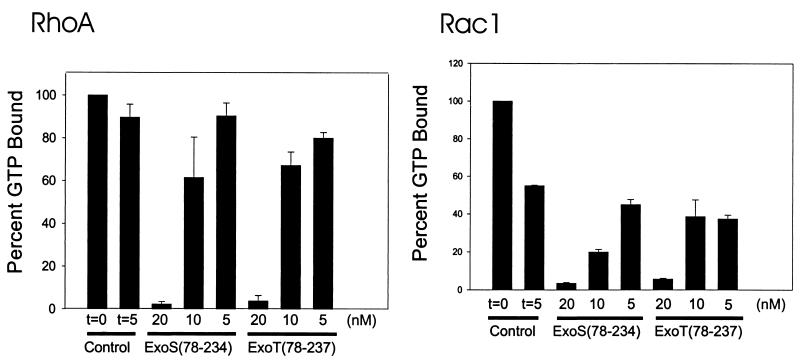
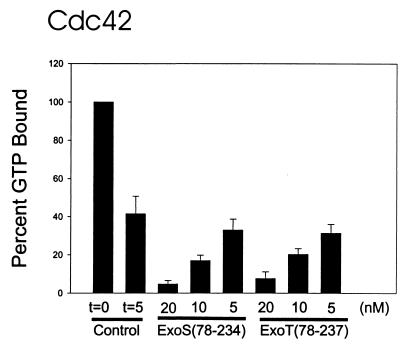
FIG. 2.
ExoS and ExoT modulation of intrinsic activity of Rho GTPases. The intrinsic GAP activities of RhoA, Rac1, and Cdc42 were determined by filter-binding assay as described in the text. Nucleotide-free Rho GTPases were loaded with [γ-32P]GTP for 5 min at 37°C. The amount of GTP bound initially (t = 0) was determined, and intrinsic GTPase activity was initiated with the addition of MgCl2. After 5 min, the amount of GTP remaining bound to the Rho GTPase was determined, and the results are presented here as percentages of the amount of GTP bound initially (100%). The amount of GTP bound in the presence of the indicated amount of ExoS(78-234) or ExoT(78-237) was also determined after the addition of MgCl2. After 5 min, the amount of GTP remaining bound to the Rho GTPase was determined, and the results are presented here as percentages of the amount of GTP bound initially (100%). Typical GTP loading of RhoA was 0.6 to 1.0 mol of GTP bound per mole of RhoA. The data are means + standard deviations (error bars).