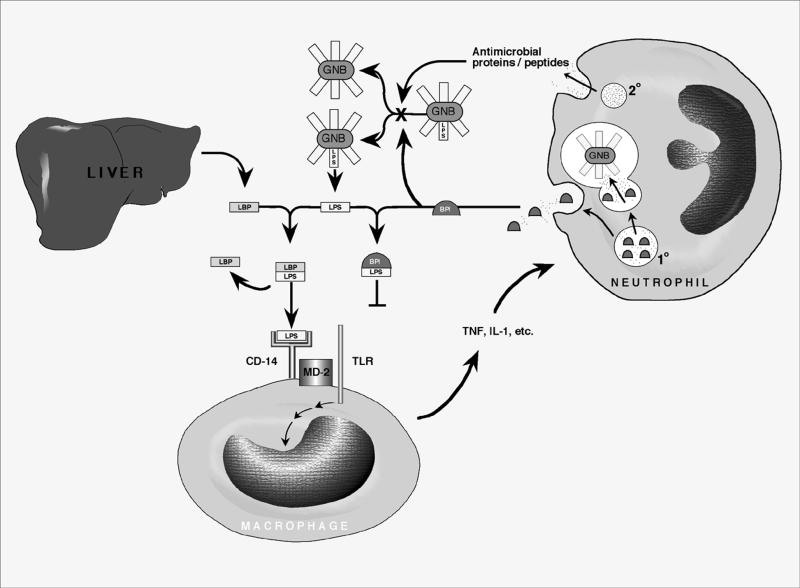
FIG. 3.
Mechanisms of antibacterial and antiendotoxic activities of neutrophils against gram-negative bacteria (GNB). The presence of gram-negative bacteria in normally sterile body compartments is signaled by bacterial surface LPS (endotoxin). LPS is specifically bound by the plasma LBP, a plasma constituent which is secreted by the liver. The LBP-LPS complex is recognized by the macrophage CD-14 receptor, which, via an adaptor protein such as MD-2, activates a Toll-like receptor protein (TLR) that transduces a signal leading to secretion of proinflammatory mediators including tumor necrosis factor (TNF) and interleukin 1 (IL-1). Neutrophils migrate to sites of infection, where they deploy their granule-associated antimicrobial arsenal both intracellularly within the phagolysosome and extracellularly by degranulation. Antimicrobial proteins and peptides from both compartments participate in bacterial growth inhibition. The role of BPI is highlighted in that it binds to LPS with a high affinity, thereby preventing formation of the LBP-LPS complex and markedly decreasing the proinflammatory effects of endotoxin.