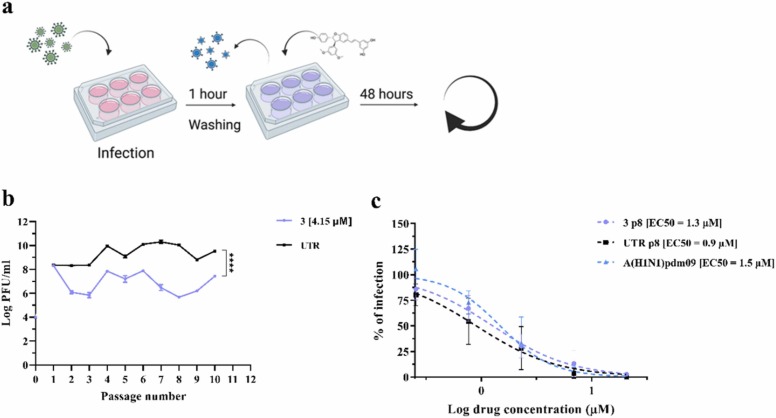
Fig. 4.
Assessment of the genetic barrier to resistance of A(H1N1)pdm09 against 3. (a) A schematic representation of the resistance assay, created with BioRender.com. (b) Comparison of viral titers from supernatants collected from Calu-3 cells infected with A(H1N1)pdm09 for 48 h in the presence of 2.1 µM of 3 in the first passage and 4.2 µM from the second and follow up passages. The titer (plaque assay performed in MDCK cells) is expressed in plaque-forming units per milliliter (log PFU/ml). The results represent the mean and SD of the Area Under the Curve (AUC) from two independent titrations performed in duplicate. Statistical significance was calculated using a two-way ANOVA analysis: * ** *, p ≤ 0.0001. (c) Antiviral effect of 3 against A(H1N1)pdm09 variants grown for eight passages in Calu-3 cells and measured by a dose-response assay in MDCK cells. Mean EC50 values and SD were calculated from two independent experiments performed in duplicate. 3 p8 = virus passaged eight times in the presence of 3; UTR p8 = virus passaged eight times in absence of drug; A(H1N1)pdm09 Stock = virus produced in MDCK cells and never passaged in Calu-3.