Abstract
True thymic hyperplasia is defined as an increase in both the size and weight of the gland, while maintaining a normal microscopic architecture. Massive true thymic hyperplasia is a rare type of hyperplasia that compresses adjacent structures and causes various symptoms.
Limited reports address the imaging findings of massive true thymic hyperplasia. Herein, we report a case of massive true thymic hyperplasia in a 3-year-old girl with no remarkable medical history. Contrast-enhanced CT revealed an anterior mediastinal mass with a bilobed configuration containing punctate and linear calcifications in curvilinear septa, which corresponded to lamellar bone deposits in the interlobular septa. To our knowledge, this is the first report of massive true thymic hyperplasia with osseous metaplasia. We also discuss the imaging features and etiology of massive true thymic hyperplasia with osseous metaplasia.
Keywords: Massive true thymic hyperplasia, Osseous metaplasia
Introduction
Massive true thymic hyperplasia is a rare variant of true thymic hyperplasia, with only approximately 50 cases reported to date [1]. Most cases occur between the ages of 1 and 15 years [1]. Due to its rarity, the imaging findings are largely unknown. Herein, we report the case of 3-year-old girl with massive thymic hyperplasia and osseous metaplasia. To our knowledge, this is the first report of massive true thymic hyperplasia with osseous metaplasia. We also present the pathological/radiologic correlation and discuss the imaging features and etiology.
Case report
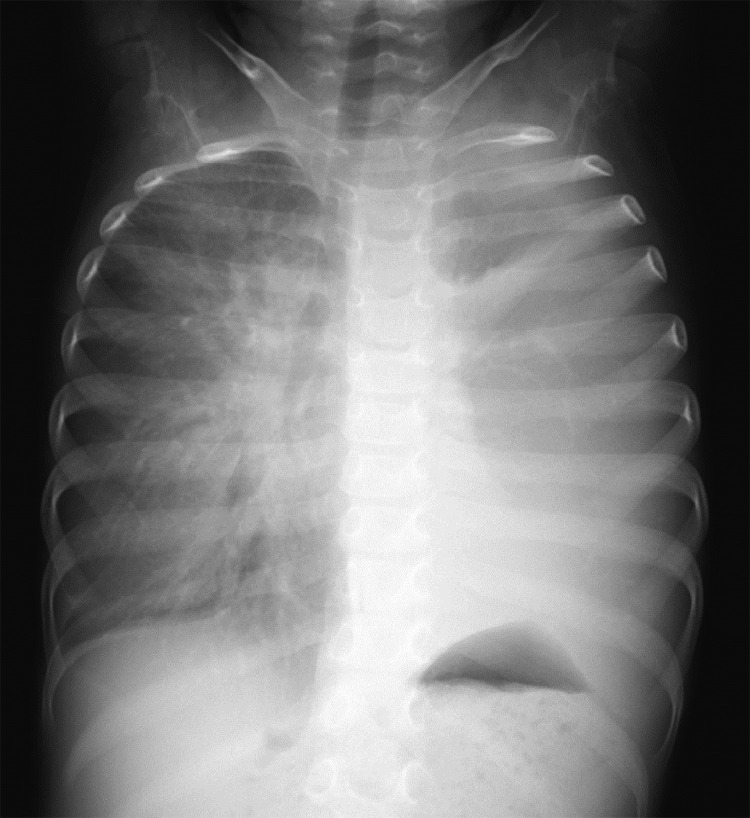
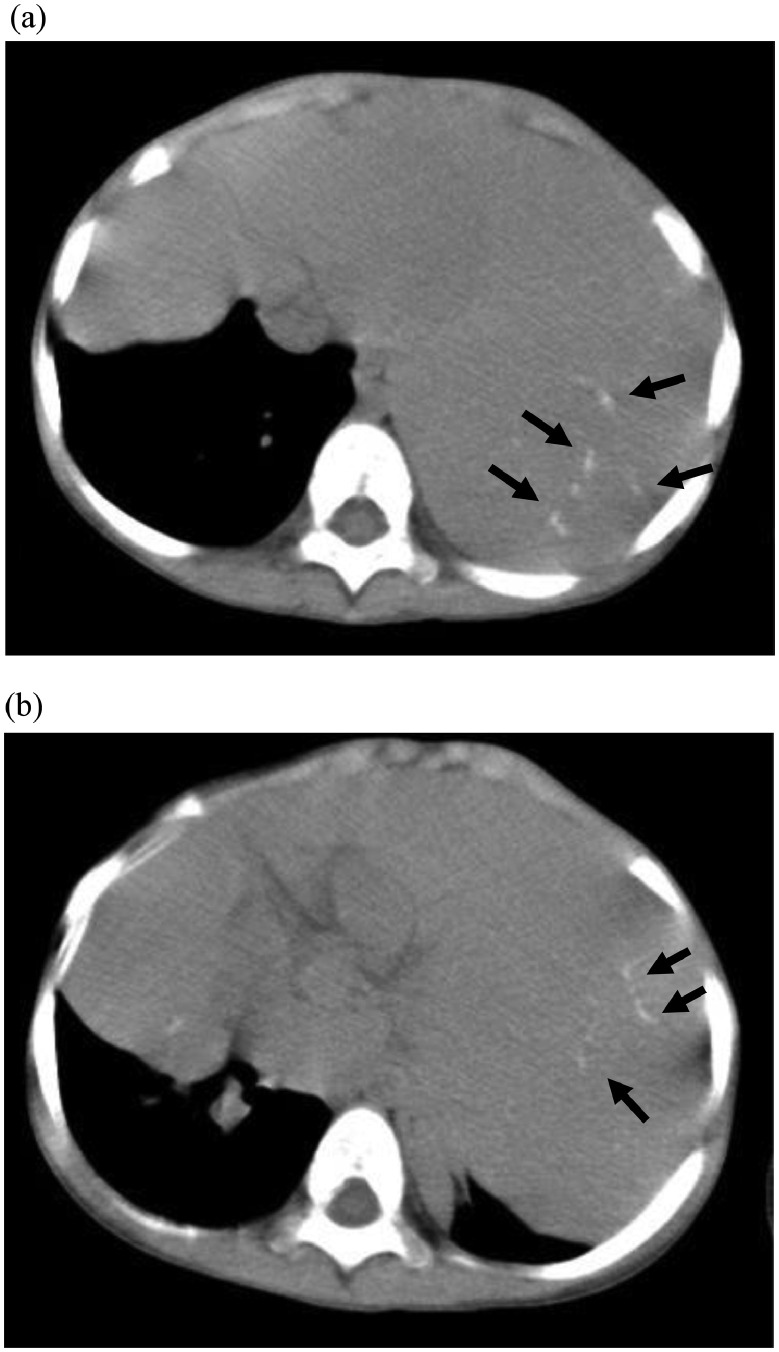
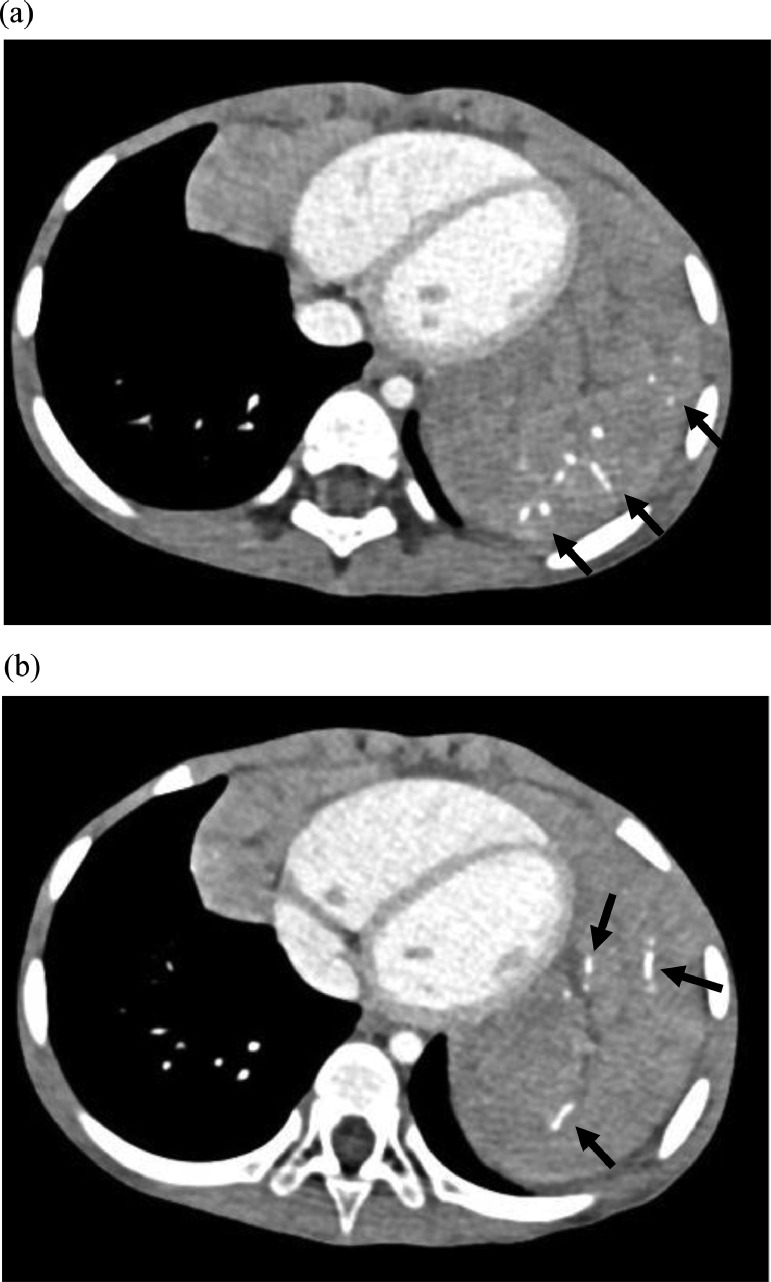
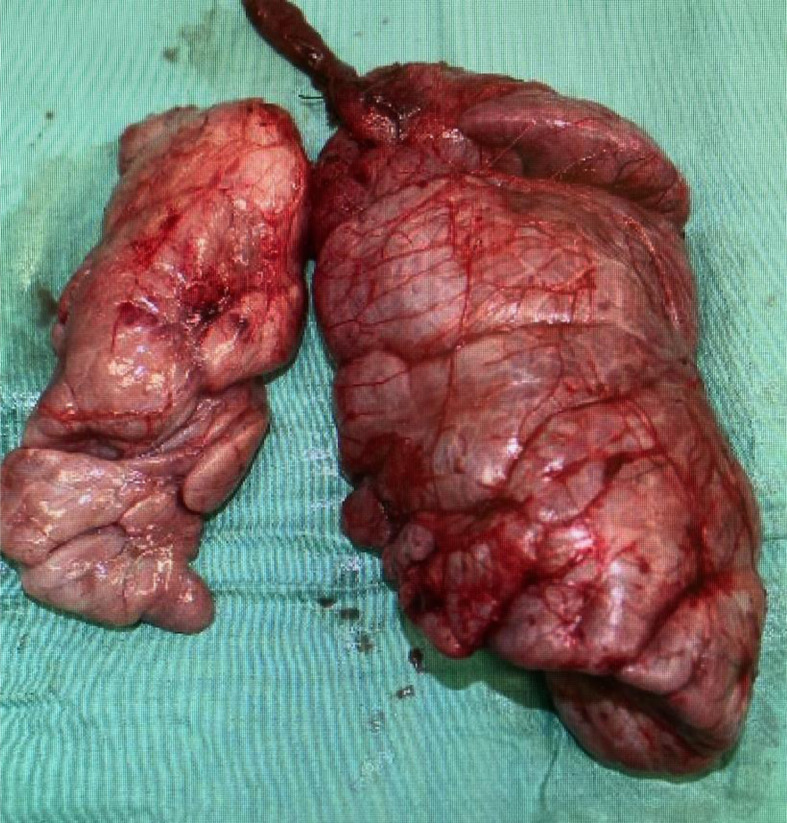
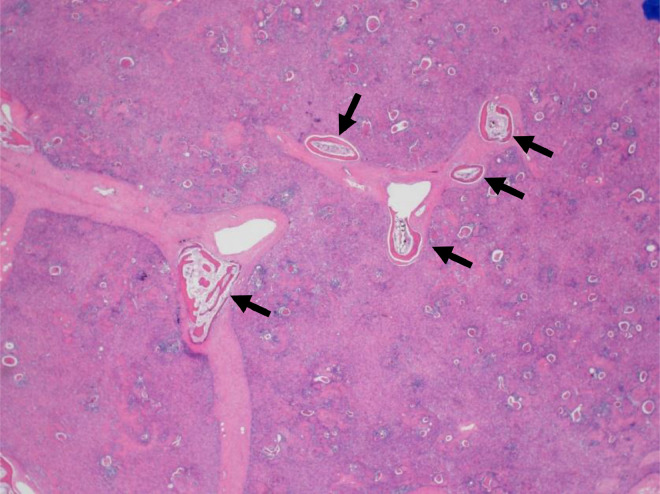
A 3-year-old girl was referred to our hospital with a complaint of dyspnea for 3 days. Her medical and familial histories were unremarkable. On initial examination, she had tachypnea, retractive breathing, and low oxygen saturation. The patient was afebrile and fully conscious. Laboratory results were as follows: white blood cell count, 13,600/mm3, hemoglobin 12.0 g/dL, platelets 268,000/mm3; C-reactive protein 0.01 mg/dL, procalcitonin 0.05 ng/mL, lactate dehydrogenase 311 U/L (>245 U/L), and soluble interleukin-2 receptor 1080 U/mL (>496 U/mL). The urine findings were normal: VMA 7.8 mg/L, HVA 7.3 mg/L. Chest radiography revealed a large mass in the mediastinum (Fig. 1). Unenhanced CT images revealed a large anterior mediastinal mass with linear and punctate calcifications (Fig. 2). Emergency irradiation (4 Gy/2 fractions) under strict respiratory management was performed for palliative purposes. Contrast-enhanced CT on the 11th day showed that the mass had slightly decreased in size. The mass had a bilobed configuration with linear and punctate calcifications in curvilinear septa (Fig. 3). Consequently, true thymic hyperplasia was suspected. On the 14th day, surgical resection was performed. Macroscopically, the mass appeared to be an enlarged thymus that retained its original structure (Fig. 4). Histologically, the specimen was composed of hyperplastic thymic tissue with a preserved lobular pattern (Fig. 5). The interlobular septa were thickened and contained lamellar bone deposits (Fig. 5). Resultantly, the patient was diagnosed with true thymic hyperplasia with osseous metaplasia. Her symptoms improved postoperatively, and the postoperative course was uneventful.
Fig. 1.
Chest radiograph. Chest radiography on admission reveals a large mass extending from the left to the right hemithorax.
Fig. 2.
Unenhanced CT images (A), (B). Unenhanced CT images show a large anterior mediastinal mass with linear and punctate calcifications (arrows).
Fig. 3.
Contrast-enhanced CT images (A), (B). Contrast-enhanced CT images show a bilobed mass in the anterior mediastinum and linear and punctate calcifications arranged in curvilinear septa (arrows). Consequently, thymic hyperplasia was suspected.
Fig. 4.
Surgical specimen. Surgical specimen demonstrates an enlarged thymus retaining its original structure.
Fig. 5.
Histopathology specimen with hematoxylin and eosin staining. Histopathological examination of the specimen reveals hyperplastic thymic tissue with preserved lobular pattern. Lamellar bone deposits were found in the thickened interlobular septa (arrows).
Discussion
Thymic hyperplasia consists of 2 separate types: true hyperplasia and follicular hyperplasia [2]. True hyperplasia is defined as thymic enlargement, retaining normal microscopic structures [2]. Rebound thymic enlargement after initial atrophy caused by stress, such as infection and systemic treatment, is true thymic hypertrophy, which is called rebound hyperplasia [2]. Some cases are associated with systemic disorders, such as hyperthyroidism, sarcoidosis, and pure red cell aplasia, while others have an unknown etiology [3]. Follicular hyperplasia is characterized by an increased number of lymphoid follicles, which does not necessarily indicate enlargement of the thymus [2]. Various autoimmune disorders, such as myasthenia gravis, connective tissue disease, vasculitis, and hyperthyroidism are reportedly associated with follicular hyperplasia [2].
Massive true thymic hyperplasia is a rare variant of true thymic hyperplasia. Although there is no established definition, it has been described as larger than the heart on posterior‐anterior chest radiograph, several times heavier than normal thymus of the age, or occupying more than 2% of body weight [4]. Due to its large size, massive true thymic hyperplasia can compress the adjacent structures and cause various symptoms, such as dyspnea and pneumonia [1]. The postoperative prognosis is good [4].
Although few reports have identified the imaging findings of massive true thymic hyperplasia, the morphology of the mass is characteristic. Massive true thymic hyperplasia, which retains the microscopic structures of the normal thymus, shows a bilobed configuration resembling the original thymus [4]. Arliss et al [5] reported a case of massive true thymic hyperplasia with curvilinear septa and fat on contrast-enhanced CT, which corresponded to interlobular septa containing fatty deposits. In this case, the characteristic curvilinear septa with calcifications were identified on contrast-enhanced CT. Calcifications in the curvilinear septa corresponded to osseous metaplasia of the interlobular septa. To our knowledge, calcifications or ossifications have not been previously reported in true thymic hyperplasia.
Although the exact etiology remains to be elucidated, hypoxic environment can be a cause of osseous metaplasia [6]. Rapid enlargement of the thymus may cause an internal hypoxic environment and increase production of hypoxia-inducible factor 1-alpha (HIF-1α). This transcriptional regulator increases the expression of vascular endothelial growth factor, which plays an important role in angiogenesis. HIF-1α also upregulates bone morphogenetic protein-2 signaling, which can recruit mesenchymal stem cells via newly formed vessels to differentiate into osseous tissue.
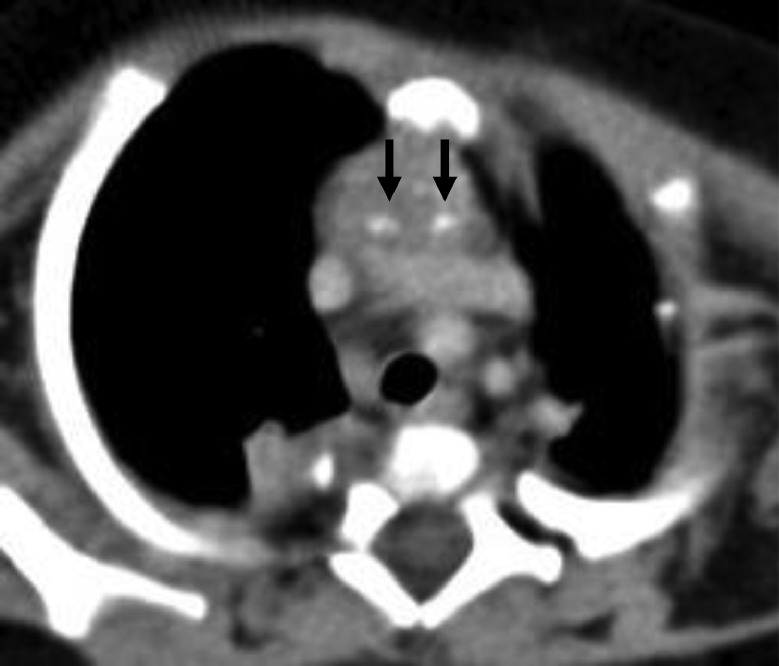
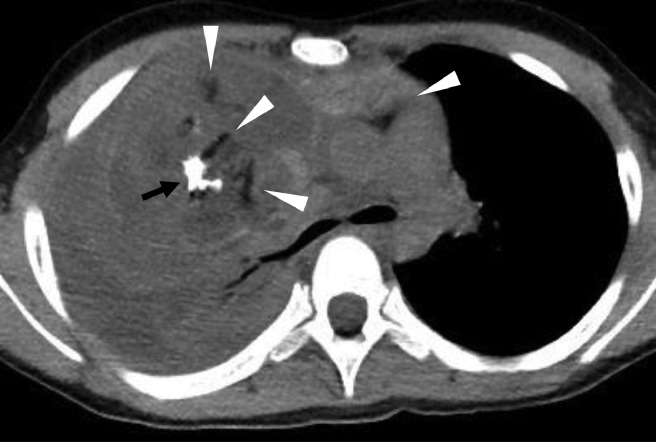
The differential diagnoses of anterior mediastinal masses with calcifications in children include Langerhans cell histiocytosis (LCH) and mature teratoma. The shape and distribution of calcifications as well as the configuration of the mass are useful in differentiating the diagnosis. LCH can affect any part of the body but is particularly common in the bone, skin, lung, and pituitary [7]. Most patients also have systemic disease at the time of thymus involvement; hence, solitary thymus lesions are rare [8,9]. Moreover, thymic LCH exhibits mostly punctate calcifications with a random distribution in the mass on CT [10] (Fig. 6). Mature teratomas typically contain fat, fluid, and calcification [11]. They exhibit rounded and discrete fatty components and dense calcifications with random distribution [5,11,12] (Fig. 7).
Fig. 6.
Thymic Langerhans cell histiocytosis: 2-month-old girl. Unenhanced CT image shows an anterior mediastinal mass with randomly distributed punctate calcifications (arrows).
Fig. 7.
Mature teratoma in the anterior mediastinum: 11-year-old girl. Unenhanced CT image shows an anterior mediastinal mass with discrete fatty components (arrowheads) and dense calcifications (arrows).
Conclusion
We have reported on a case of massive true thymic hyperplasia with osseous metaplasia. Bilobed configuration of the mass and linear and punctate calcifications arranged in curvilinear septa are characteristic findings. Osseous metaplasia may be caused by an internal hypoxic environment.
Patient consent
Written informed consent was obtained from the patient's parents.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Tadiotto E, Clemente M, Pecoraro L, Piacentini G, Degani D, Pietrobelli A. Massive thymic hyperplasia in a 15-month-old boy: case report and literature review. Clin Case Rep. 2019;7:27–31. doi: 10.1002/ccr3.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manchanda S, Bhalla AS, Jana M, Gupta AK. Imaging of the pediatric thymus: clinicoradiologic approach. World J Clin Pediatr. 2017;6:10. doi: 10.5409/wjcp.v6.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nasseri F, Eftekhari F. Clinical and radiologic review of the normal and abnormal thymus: pearls and pitfalls. Radiographics. 2010;30:413–428. doi: 10.1148/rg.302095131. [DOI] [PubMed] [Google Scholar]
- 4.Linegar AG, Odell JA, Fennell WMP, Close PM, de Groot MK, Casserly DR, et al. Massive thymic hyperplasia. Ann Thorac Surg. 1993;55:1197–1201. doi: 10.1016/0003-4975(93)90033-E. [DOI] [PubMed] [Google Scholar]
- 5.Arliss J, Scholes J, Dickson PR, Messina JJ. Massive thymic hyperplasia in an adolescent. Ann Thorac Surg. 1988;45:220–222. doi: 10.1016/S0003-4975(10)62443-5. [DOI] [PubMed] [Google Scholar]
- 6.Ranganathan K, Loder S, Agarwal S, Wong VW, Forsberg J, Davis TA, et al. Heterotopic ossification: basic-science principles and clinical correlates. J Bone Joint Surg Am. 2014;97:1101–1111. doi: 10.2106/JBJS.N.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856–868. doi: 10.1056/NEJMra1607548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakatos K, Herbrüggen H, Pötschger U, Prosch H, Minkov M. Radiological features of thymic langerhans cell histiocytosis. Pediatr Blood Cancer. 2013;60:E143–E145. doi: 10.1002/pbc.24640. [DOI] [PubMed] [Google Scholar]
- 9.Lee BH, George S, Kutok JL. Langerhans cell histiocytosis involving the thymus. A case report and review of the literature. Arch Pathol Lab Med. 2003;127:e294–e297. doi: 10.5858/2003-127-e294-LCHITT. [DOI] [PubMed] [Google Scholar]
- 10.Heller GD, Haller JO, Berdon WE, Sane S, Kleinman PK. Punctate thymic calcification in infants with untreated Langerhans’ cell histiocytosis: report of four new cases. Pediatr Radiol. 1999;29:813–815. doi: 10.1007/s002470050702. [DOI] [PubMed] [Google Scholar]
- 11.Ranganath SH, Lee EY, Restrepo R, Eisenberg RL. Mediastinal masses in children. Am J Roentgenol. 2012;198:W197–W216. doi: 10.2214/AJR.11.7027. [DOI] [PubMed] [Google Scholar]
- 12.Moeller KH, Rosado-de-Christenson ML, Templeton PA. Mediastinal mature teratoma: imaging features. AJR Am J Roentgenol. 1997;169:985–990. doi: 10.2214/ajr.169.4.9308448. [DOI] [PubMed] [Google Scholar]