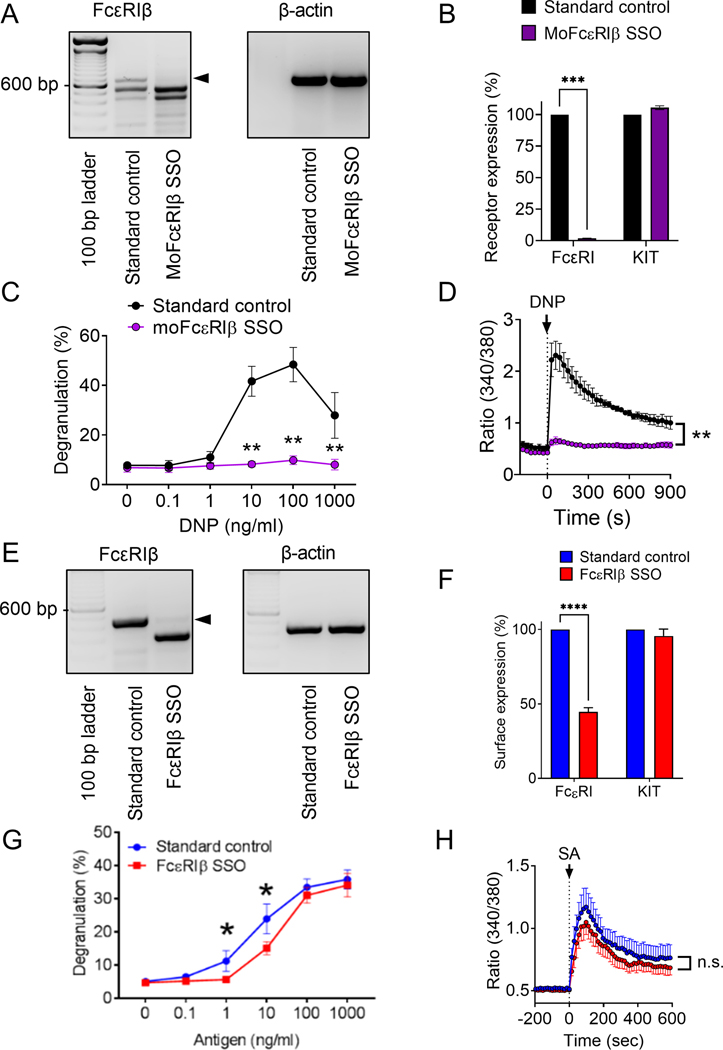
Figure 1: Surface expression of FcεRIα and degranulation of human MCs is not dependent upon the presence of FcεRIβ in the FcεRI receptor complex.
A-D Mouse BMMCs were treated with MoFcεRIβ splice-switching oligonucleotide (SSO). A) Qualitative RT-PCR of FcεRIβ and β-actin. B) Flow cytometric analysis of surface expression of Kit and FcεRIα upon treatment with standard control oligonucleotide (black) and FcεRIβ SSO (purple). C) BMMC degranulation upon stimulation with DNP following treatment with a standard control oligonucleotide and FcεRIβ SSO. D) Ratiometric calcium signaling following stimulant addition at the arrowhead. E-H) LAD2 MCs were treated with FcεRIβ SSO, termed FcεRIβ SSO, at 10 μM to induce skipping of FcεRIβ at exon 3. (E) Qualitative RT-PCR of FcεRIβ showing expression of the full length FcεRIβ variant when treated with a standard control oligonucleotide and the shorter truncated FcεRIβ variant after successful exon skipping; (F) Flow cytometric analysis of surface expression of KIT and FcεRIα upon treatment with standard control oligonucleotide (blue) and FcεRIβ SSO (red); (G) LAD2 cell degranulation upon stimulation with streptavidin (antigen) following treatment with a standard control oligonucleotide and FcεRIβ SSO; (H) Ratiometric calcium signaling following stimulant addition at the arrowhead. Data are the mean ± SEM from three experiments. *P <0.05, **P <0.01, ****P< 0.0001, n.s. = not significant, paired t-test (B & F), or ANOVA with post-test (C, D, G & H).