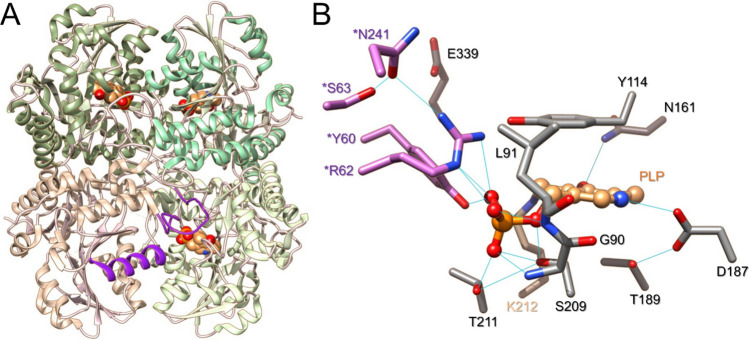
Figure 1.
Structure of cystathionine γ-lyase. (A) Structure of CSE showing its tetrameric organization (PDB code: 2NMP). Each subunit of CSE is color-coded differently. The PLP cofactor is shown in brown. Structural elements from the adjacent B subunit (loop44–65 and α-helix229–244) contributing residues to the active site of the A subunit are color-coded in purple. (B) Close-up of CSE active site from the A subunit. Important amino acid residues are represented in stick representation. The PLP cofactor (brown) establishes an imine bond at its C4’ position with Lys212 (tan color) and is stabilized through various hydrogen bonds (represented in light blue lines) with surrounding amino acid residues, including residues from the adjacent B subunit (colored in orchid).