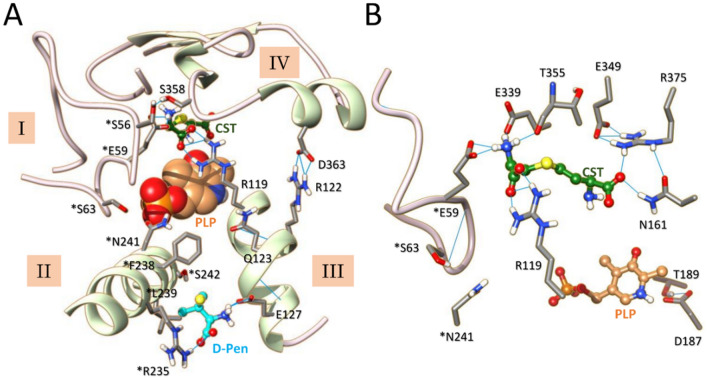
Figure 8.
Destabilization of CSE active site by D-penicillamine binding at site 33 during molecular dynamics (MD). (A, B) Representative snapshot of the active site after 50 ns MD simulations showing the impact of D-pen (cyan) binding on the hydrogen bonding network partly implicated in the unity of the substrate access channel (A) and the cystathionine interacting network (B). Significantly, binding of D-pen to the interfacial ligand-binding site located between the α-helices from structural elements II and III causes significant disturbances in the opening of the substrate access channel (A) and important variations at the active site, such as the stabilization of the distal ammonium group of cystathionine by H-bonding with *Glu59 and Glu339 and the disruption of the H-bond contact between the pyridine ring of the cofactor and Asp187 (B). *Residues from the adjacent subunit. H-bonding are depicted by light blue lines. CST, cystathionine.