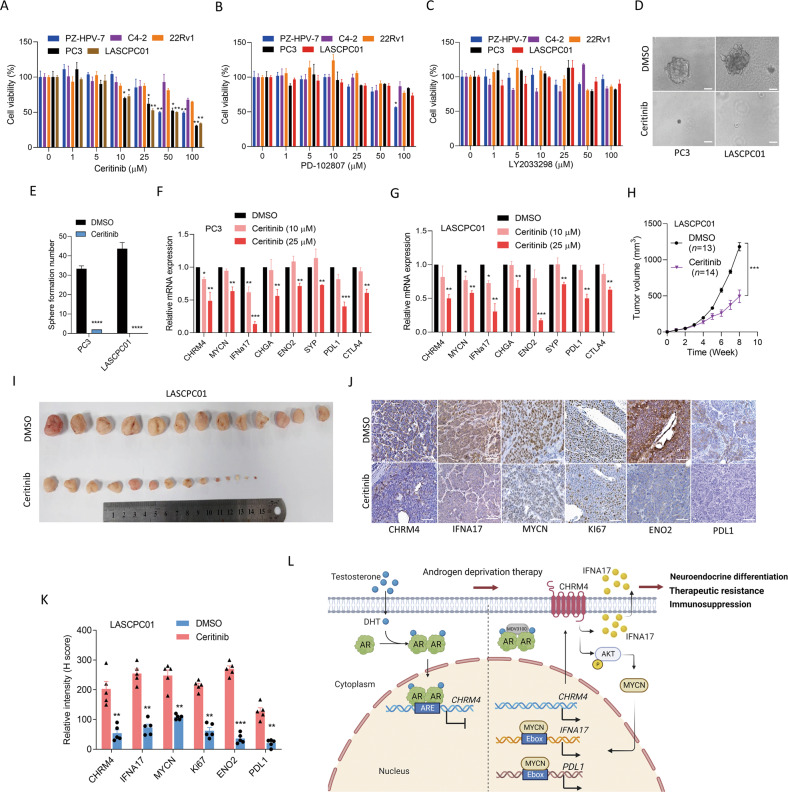
Fig. 7. Target CHRM4 reduces the tumor growth and neuroendocrine differentiation of prostate cancer.
Various prostate cancer cells were treated with 0, 1, 5, 10, 25, 50, and 100 μM of the small-molecule drugs, ceritinib (A), PD102807 (B), and LY2033298 (C) for 24 h, and cell viability was assessed by an MTT colorimetric assay. * vs. the control (0 μM). n = 8 per group. D, E Sphere-formation assay of PC3 and LASCPC01 cells treated with DMSO or 5 μM ceritinib during 1 week. * vs. DMSO. n = 5 per group by a t-test. F, G Relative CHRM4, MYCN, IFNA17, NE marker (CHGA, ENO2, and SYP), and immune checkpoint (PDL1 and CTLA4) mRNA levels in PC3 and LASCPC01 cells treated with DMSO or ceritinib at 10 and 25 μM for 24 h, as measured by an RT-qPCR analysis. * vs. DMSO, by a one-way ANOVA. Quantification of relative mRNA expressions is presented as the mean ± SEM from three biological replicates. *p < 0.05, **p < 0.01, ***p < 0.001. H, I Tumor growth monitoring of LASCPC01 cells subcutaneously injected into male nude mice. One month after injection, DMSO or ceritinib (25 mg/kg) was intraperitoneally inoculated into mice once a week for 4 weeks. The tumor volume was measured every week, and tumor tissues were collected on the last day of the experiment. DMSO-injected mice (n = 13); ceritinib-injected mice (n = 14). * vs. DMSO, ***p < 0.001, by a t-test. IHC staining (J) and representative intensities (K) of CHRM4, IFNA17, MYCN, KI67, ENO2, and PDL1 in subcutaneous tumors from I. * vs. DMSO. **p < 0.01, ***p < 0.001. Significance was examined by a two-tailed Student’s t-test. L A schematic summary of this study. Our study focused on androgen-deprivation therapy (ADT)-induced NE prostate cancer (NEPC) to determine the mechanism by which androgen receptor (AR) loss of function might promote CHRM4-driven AKT/MYCN signaling leading to increased IFNA17 and PDL1 expressions. Increased abundances of IFNA17 and PDL1 may be regulated by the MYCN transcription factor through a positive feedback mechanism. Serum IFNA17 levels can be considered a prognostic biomarker in NEPC-like prostate cancer, and targeting CHRM4 may have the potential to inhibit NEPC progression.