Abstract
Background
The term “multimorbidity” identifies high-risk, complex patients and is conventionally defined as ≥2 comorbidities. However, this labels almost all older patients as multimorbid, making this definition less useful for physicians, hospitals, and policymakers.
Objective
Develop new medical condition-specific multimorbidity definitions for patients admitted with acute myocardial infarction (AMI), heart failure (HF), and pneumonia patients. We developed three medical condition-specific multimorbidity definitions as the presence of single, double, or triple combinations of comorbidities — called Qualifying Comorbidity Sets (QCSs) — associated with at least doubling the risk of 30-day mortality for AMI and pneumonia, or one-and-a-half times for HF patients, compared to typical patients with these conditions.
Design
Cohort-based matching study
Participants
One hundred percent Medicare Fee-for-Service beneficiaries with inpatient admissions between 2016 and 2019 for AMI, HF, and pneumonia.
Main Measures
Thirty-day all-location mortality
Key Results
We defined multimorbidity as the presence of ≥1 QCS. The new definitions labeled fewer patients as multimorbid with a much higher risk of death compared to the conventional definition (≥2 comorbidities). The proportions of patients labeled as multimorbid using the new definition versus the conventional definition were: for AMI 47% versus 87% (p value<0.0001), HF 53% versus 98% (p value<0.0001), and pneumonia 57% versus 91% (p value<0.0001). Thirty-day mortality was higher among patients with ≥1 QCS compared to ≥2 comorbidities: for AMI 15.0% versus 9.5% (p<0.0001), HF 9.9% versus 7.0% (p <0.0001), and pneumonia 18.4% versus 13.2% (p <0.0001).
Conclusion
The presence of ≥2 comorbidities identified almost all patients as multimorbid. In contrast, our new QCS-based definitions selected more specific combinations of comorbidities associated with substantial excess risk in older patients admitted for AMI, HF, and pneumonia. Thus, our new definitions offer a better approach to identifying multimorbid patients, allowing physicians, hospitals, and policymakers to more effectively use such information to consider focused interventions for these vulnerable patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-022-07897-4.
Keywords: multimorbidity, Medicare, inpatient, AMI, heart failure, pneumonia
INTRODUCTION
Multimorbidity refers to the coexistence of multiple chronic conditions which is common among adults and typical among older adults.1 It has been defined in the general population by various methods2–9 and datasets, most commonly administrative claims data.10–15 Conventionally, studies have defined multimorbidity as a specific number of patient comorbidities, usually two or more.2,16–25 The usefulness of this conventional two-or-more comorbidities definition is uncertain in hospitalized medical patients because such patients are far sicker than the general population. Furthermore, the conventional definition does not distinguish specific combinations of comorbidities but is simply based on the number of comorbidities.
Multimorbid patients are typically at a high risk of death, and so model-based scores that evaluate the risk of death are highly relevant to multimorbidity.26–31 Nonetheless, multimorbid patients are heterogeneous and two multimorbid patients with the same high risk may be high-risk for very different reasons. An important goal of our work is to make useful distinctions among high-risk multimorbid patients.
In this study, we will screen and validate a vast number of comorbidity combinations to identify a modest number of common high-risk comorbidity combinations called Qualifying Comorbidity Sets (QCSs) useful in health outcomes analyses. These QCSs, when present, will label patients as “multimorbid.” We developed QCSs for three common principal diagnoses of inpatient stays in the USA,32 acute myocardial infarction (AMI), heart failure (HF), and pneumonia.
METHODS
Patient Population
We used Medicare claims (Inpatient, Outpatient, Carrier/Part B, Skilled Nursing Facility, Home Health Agency, and Durable Medical Equipment (DME) files) for all fee-for-service Medicare beneficiaries through the Centers for Medicare and Medicaid Services (CMS) Virtual Research Data Center.33 For creating our multimorbidity definitions, we analyzed patients aged 66 years and older who were hospitalized for AMI, HF, or pneumonia during the years 2016–2017.
AMI, HF, and pneumonia patients were categorized into clinically relevant condition groups using ICD-10 principal diagnosis codes for their index hospitalization (Appendix A, Tables A.1-A.3). For patients with multiple admissions, we used one randomly chosen admission for their index hospitalization. We excluded patients if in the 1-year lookback prior to their admission they either (1) lacked fee-for-service Medicare claims, (2) did not have complete enrollment in Medicare Parts A and B, or (3) were enrolled in an HMO at any point.
We excluded patients with metastatic cancers or Alzheimer’s disease and related dementias (ADRD) (Appendix B, Table B.1) or those aged ≥90 years (MC/ADRD/90) from the development of the multimorbidity definitions. These conditions are associated with such high mortality rates that their inclusion in our reference group would make it difficult to detect increased mortality from other comorbidities. Patients with these high-risk conditions are more likely to have different goals of care in the event of life-threatening complications, making mortality rates difficult to interpret.8 However, after we developed our multimorbidity definitions, we report on the multimorbidity status of these high-risk MC/ADRD/90 patients.
Defining Comorbidities
To define comorbidities, we utilized CMS Hierarchical Condition Category (HCC) version 22 (v22)34,35 because it includes both International Classification of Diseases Clinical Modification, 10th Revision (ICD-10)36 and 9th Revision (ICD-9)37 codes. We also used HCCs from the CMS RxHCC model.38 To better capture patient health status,39–42 we considered functional status indicators from CMS DME files in order to determine whether using these indicators improved our model predictions.
We analyzed 53 variables in total, 51 comorbidities—derived from 89 HCCs (Appendix E)—plus 2 functional status variables (Appendix D, Table D.1). Details of these variables with diagnostic codes are described in Appendix E, Table E.1.
All comorbidities and functional status indicators were identified in 1-year lookback periods. We also identified these indicators at the index hospitalization encounters. However, during the index hospitalizations, certain conditions can be a result of in-hospital complications; hence, we used them as comorbidities only if they were marked as present on admission (POA), see Appendix E, Table E.3.
Patients with multiple comorbidities within a clinically relevant group were only assigned the most severe comorbidity (Appendix E, Table E.2). For example, a patient with history of both “diabetes with complications” and “diabetes without complications” was only assigned “diabetes with complications”.
Defining Functional Status
We tested various functional status variables from the CMS DME files and identified two classes of indicators:39–42 (1) need for home oxygen supplementation43,44 and (2) need for a home hospital bed or wheelchair.43–45 Details of the CPT/HCPCS codes are provided in Appendix D, Table D.1. Other functional status indicators produced no added benefit in modelling (Appendix D, Table D.2).
Defining Multimorbidity
A patient was defined as multimorbid if they had at least one QCS from the lists of the three medical condition-specific QCSs. A QCS was defined as a single, double, or triple combination of comorbidities or functional status variables that, when present, at least doubled the odds of mortality for AMI and pneumonia patients or increased the odds of mortality by a factor of 1.5 for HF patients compared to typical patients with these conditions.8 The mortality was observed within 30-day46–49 from index admission.
For HF, we used a lower cut-off because patients admitted for HF typically have far more comorbidities than AMI or pneumonia patients, and it was therefore rare for a QCS to double the risk of 30-day mortality for HF patients relative to the HF reference group. When we used 2-fold risk of mortality risk as a threshold, we found that only 15.7% of HF patients were labeled as multimorbid, whereas when we used 1.5-fold risk threshold, about half of HF patients were labeled as multimorbid.
The study used two non-overlapping datasets for index admissions. The first dataset was for the development of the multimorbid definition comprised of admissions in 2016–2017. The second dataset was used to validate the definitions and comprised of admissions in 2018–2019.
First, for development, we used 2016–2017 admissions for AMI, HF, and pneumonia to identify QCSs. There were 24,857 possible single, double or triple combinations of 51 comorbidities plus 2 functional status indicators, from which we derived a shorter list of QCSs. To avoid mistakenly finding QCSs by chance due to multiple testing, we utilized cross-screening for validation where the dataset was split randomly into two halves. The process of screening a QCS was done in the first half and validated in the second half. Then it was repeated by screening in the second half and validating in the first half. Any QCS validated in either half was included in our definition. The details are described in our previous work.8,50 We used the Mantel-Haenszel test51 in each random half of the data stratified by medical conditions groups (four for AMI, four for HF, eight for pneumonia), and three age groups, 65 to 74, 75 to 84, and ≥85.
Second, for validation, we explored how our newly developed definitions performed when applied to an external dataset of 2018–2019 admissions. We made comparisons of multimorbid patient 30-day mortality by two reference populations. First, the “typical reference” group, consisted of a random 20% sample of all 2018–2019 admissions. This comparison was a more ‘real-world’ comparison, which allow us to ascertain the risk of mortality among multimorbid patients relative to typical patients. The second reference population—the “non-multimorbid”—was a subset of the typical reference group. It comprised only those typical reference group patients who do not have any QCSs i.e., non-multimorbid patients. This comparison represented the “pure” risk associated with multimorbid compared to non-multimorbid patients. Within each “Typical” and “Non-MM” reference group, patients were medical condition specific; i.e., the odds of mortality among multimorbid patients for each medical condition type were compared to the reference group patients with the same medical conditions.
External Validation of Multimorbidity Definitions
To externally validate our multimorbidity definitions, we used 2018–2019 admissions to evaluate whether multimorbid patients had higher 30-day and 90-day mortality than non-multimorbid patients and whether this varied by hospital resources. We examined this question in hospitals with characteristics generally associated with better outcomes, which we called “better-resourced” hospitals, and compared them to “other” hospitals (all hospitals that were not defined as better-resourced). We used multivariate matching52,53 to develop condition-specific matched pair sets of multimorbid patients in better-resourced hospitals with patients in other hospitals. We performed similar analyses with non-multimorbid patients. Finally, we examined the difference-in-differences between multimorbid versus non-multimorbid patients. We have detailed our matching technique in prior works.54,55 We used methods for paired binary data for difference-in-difference analyses.56
To define “better-resourced” hospitals, we used an external dataset of medical admissions for all Medicare beneficiaries between January 1, 2012, and September 30, 2015, to create risk-adjusted models for 30-day mortality incorporating patient and hospital characteristics (Appendix C, Tables C.1-C.2). We described better-resourced hospitals as having all three of the following characteristics: (1) resident-to-bed ratio >0.25 (suggestive of major and very major teaching hospitals), (2) nursing skill mix above the median, and (3) condition-specific patient volume above the median (Appendix C, Table C.3). The distribution of hospital characteristics is presented in Appendix C, Table C.4.
The study has been approved by the Institutional Review Board of the Children’s Hospital of Philadelphia. All analyses were completed using SAS version 9.4.57
RESULTS
Examining AMI, HF, and Pneumonia Patients
For AMI patients, we identified 50 QCSs (including 6 singles, 20 doubles, and 24 triples), that at least doubled the odds of mortality compared to the typical reference group. For HF patients, we found 118 QCSs (3 singles, 24 doubles, and 91 triples) that increased the odds of dying by 50%. Among pneumonia patients, a total of 51 QCSs (4 singles, 12 doubles, and 35 triples) doubled the odds of death. In contrast, when multimorbidity was defined by the conventional definition of the presence of ≥2 comorbidities, there were 1265 observed combinations (i.e., every possible combination per the conventional definition was observed for each medical condition).
We presented the odds ratios of 30-day mortality to two reference groups: (1) ORnon represents odds ratios compared to the non-multimorbid reference and (2) ORtypical represents odds ratios compared to the typical reference (comprised of both multimorbid and non-multimorbid patients). Roughly half the typical patients were multimorbid, thus ORtypical was always much smaller than ORnon. As expected, the odds of death were much higher for every QCS when compared to the non-multimorbid reference group than the typical reference group. For AMI patients, the ORnon was 6.99 (95% CI 6.44, 7.58) and ORtypical was 2.17 (2.09, 2.26); for HF ORnon was 2.97 (2.80, 3.15) and ORtypical was 1.47 (1.42. 1.52); and for Pneumonia ORnon was 5.17 (4.91, 5.44) and ORtypical was 1.65 (1.62, 1.69). The most common QCSs and the QCSs with the highest odds of 30-day mortality for AMI, HF, and pneumonia are described below and are shown in Tables 1, 2 and 3, respectively; full lists of QCSs are provided in Appendix G, Tables G.1-G.3.
Table 1.
Frequency and Odds of 30-Day Mortality with Qualifying Comorbidity Sets for Acute Myocardial Infarction Patients
| Qualifying Comorbidity Sets* | Frequency (%) 186,036 (100.00) |
Ref. non-MM† (N=24,812) | Ref. typical† (N=44,555) |
||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
| A. Top 10 QCS listed by highest to lowest frequency | |||||
| Acute Heart or Respiratory Failure | 42,342 (22.76) | 10.62 | (9.78, 11.53) | 3.32 | (3.19, 3.47) |
| Heart Failure | Acute Renal Failure | 23,679 (12.73) | 8.13 | (7.46, 8.88) | 2.60 | (2.47, 2.73) |
| CKD Stage 4-5 or Dialysis | 21,134 (11.36) | 7.61 | (6.95, 8.32) | 2.46 | (2.34, 2.59) |
| Cardiac Arrhythmias | Acute Renal Failure | 16,041 (8.62) | 8.35 | (7.63, 9.14) | 2.67 | (2.53, 2.82) |
| Chronic Pulmonary Diseases | Acute Renal Failure | 14,030 (7.54) | 7.94 | (7.24, 8.72) | 2.56 | (2.41, 2.71) |
| Sepsis or Septic Shock | 12,310 (6.62) | 8.55 | (7.78, 9.39) | 2.75 | (2.59, 2.91) |
| Pneumonias | 10,691 (5.75) | 8.20 | (7.45, 9.01) | 2.65 | (2.49, 2.82) |
| Protein-Calorie Malnutrition | 10,172 (5.47) | 8.85 | (8.04, 9.73) | 2.89 | (2.72, 3.08) |
| Heart Failure | Vascular Diseases | Thrombocytopenia and Other Hematological Disorders | 9543 (5.13) | 8.19 | (7.42, 9.04) | 2.69 | (2.52, 2.87) |
| Heart Failure | Cardiac Arrhythmias | Thrombocytopenia and Other Hematological Disorders | 9159 (4.92) | 8.04 | (7.28, 8.87) | 2.63 | (2.47, 2.81) |
| B. Top 5 QCS listed by highest to lowest odds of mortality‡ | |||||
| Liver Diseases | Acute Renal Failure | 1295 (0.70) | 15.45 | (13.22, 18.06) | 4.98 | (4.35, 5.70) |
| Endocrine and Metabolic Disorders | Chronic Non-Pressure Skin Ulcers | Complications of Implants or Grafts | 1296 (0.70) | 11.37 | (9.60, 13.46) | 3.64 | (3.14, 4.21) |
| Liver Diseases | Thrombocytopenia and Other Hematological Disorders | 1733 (0.93) | 11.38 | (9.81, 13.21) | 3.58 | (3.16, 4.07) |
| Diabetes with Complications | Thrombocytopenia and Other Hematological Disorders | Complications of Implants or Grafts | 1664 (0.90) | 10.76 | (9.23, 12.55) | 3.46 | (3.04, 3.95) |
| Acute Heart or Respiratory Failure | 42,342 (22.76) | 10.62 | (9.78, 11.53) | 3.32 | (3.19, 3.47) |
| C. Patients for MM definition development and MC/ADRD/90 patients | |||||
| Patients for MM definition development | 186,036 (100.00) | ||||
| MM | 86,949 (46.74) | 6.99 | (6.44, 7.58) | 2.17 | (2.09, 2.26) |
| Non-MM | 99,063 (53.26) | -- | -- | 0.33 | (0.32, 0.35) |
| MC/ADRD/90 patients§ | 77,818 (100.00) | 7.89 | (7.26, 8.58) | 2.52 | (2.41, 2.63) |
| MM | 52,179 (67.05) | 10.68 | (9.81, 11.64) | 3.43 | (3.28, 3.58) |
| Non-MM | 25,639 (32.95) | 2.81 | (2.54, 3.12) | 0.95 | (0.90, 1.02) |
*The full list of 50 QCSs with their frequency and ORs (95% CI) are provided in Appendix G (Table G.1)
† Typical reference group consisting of a random 20% sample of 2018-2019 admissions. Patients with Metastatic Cancers, ADRD, age ≥90 years, or on dialysis were not included in the typical reference group.; Non-multimorbid reference group drawn from the same random 20% sample comprised of patients who do not have any QCSs as well as patients without Metastatic Cancers, ADRD, or age ≥90 years. Dashes (--) indicate a population odds ratio of 1, reflecting the fact that the comparison groups are a random split of one population
‡Ranked by lower 95% CI of odds for 30-day mortality relative to the medical condition-specific typical reference population
§Frequency and odds of 30-day mortality for multimorbid and non-multimorbid patients with Metastatic Cancers, ADRD, or age ≥90 years are further detailed in Appendix G (Table G.4)
Table 2.
Frequency and Odds of 30-Day Mortality for by Qualifying Comorbidity Sets for Heart Failure Patients
| Qualifying Comorbidity Sets* | Frequency (%) 314,510 (100.00) |
Ref. non-MM† (N=36,634) | Ref. typical† (N=73,702) |
||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
| A. Top 10 QCS listed by highest to lowest frequency | |||||
| Protein-Calorie Malnutrition | 32,385 (10.30) | 4.83 | (4.53, 5.15) | 2.39 | (2.29, 2.49) |
| Thrombocytopenia and Other Hematological Disorders | Acute Heart or Respiratory Failure | Heart Failure | 31,018 (9.86) | 3.84 | (3.59, 4.11) | 1.91 | (1.83, 2.00) |
| Acute Heart or Respiratory Failure | Heart Failure | Acute Myocardial Infarction | 30,717 (9.77) | 3.67 | (3.43, 3.93) | 1.81 | (1.73, 1.90) |
| Sepsis or Septic Shock | Acute Heart or Respiratory Failure | 29,982 (9.53) | 3.61 | (3.37, 3.86) | 1.79 | (1.71, 1.87) |
| Acute Heart or Respiratory Failure | Pneumonias | 29,660 (9.43) | 3.61 | (3.37, 3.86) | 1.79 | (1.71, 1.87) |
| Thrombocytopenia and Other Hematological Disorders | Acute Heart or Respiratory Failure | Coronary Artery Disease | 27,477 (8.74) | 3.80 | (3.55, 4.07) | 1.89 | (1.81, 1.98) |
| Thrombocytopenia and Other Hematological Disorders | Acute Heart or Respiratory Failure | Cardiac Arrhythmias | 27,340 (8.69) | 3.96 | (3.70, 4.23) | 1.98 | (1.89, 2.07) |
| Heart Failure | Cardiac Arrhythmias | Pneumonias | 26,873 (8.54) | 3.46 | (3.23, 3.70) | 1.72 | (1.64, 1.81) |
| Acute Heart or Respiratory Failure | Cardiac Arrhythmias | CKD Stage 4-5 or Dialysis | 26,556 (8.44) | 3.81 | (3.53, 4.12) | 1.84 | (1.75, 1.93) |
| Acute Heart or Respiratory Failure | Chronic Pulmonary Diseases | CKD Stage 4-5 or Dialysis | 26,183 (8.33) | 3.48 | (3.21, 3.76) | 1.67 | (1.59, 1.76) |
| B. Top 5 QCS listed by highest to lowest odds of mortality‡ | |||||
| Thrombocytopenia and Other Hematological Disorders | Chronic Non-Pressure Skin Ulcers | Home Oxygen Use | 2394 (0.76) | 4.93 | (4.34, 5.59) | 2.44 | (2.17, 2.73) |
| Protein-Calorie Malnutrition | 32,385 (10.30) | 4.83 | (4.53, 5.15) | 2.39 | (2.29, 2.49) |
| Thrombocytopenia and Other Hematological Disorders | Home Hospital Bed or Wheelchair use | Home Oxygen Use | 1713 (0.55) | 4.82 | (4.16, 5.58) | 2.38 | (2.08, 2.73) |
| Thrombocytopenia and Other Hematological Disorders | Acute Heart or Respiratory Failure | Chronic Non-Pressure Skin Ulcers | 6210 (1.98) | 4.79 | (4.36, 5.26) | 2.37 | (2.20, 2.56) |
| Thrombocytopenia and Other Hematological Disorders | CKD Stage 4-5 or Dialysis | Home Oxygen Use | 4248 (1.35) | 4.75 | (4.26,5.30) | 2.30 | (2.10,2.51) |
| C. Patients for MM definition development and MC/ADRD/90 patients | |||||
| Patients for MM definition development | 314,510 (100.00) | ||||
| MM | 167,739 (53.33) | 2.97 | (2.80, 3.15) | 1.47 | (1.42, 1.52) |
| Non-MM | 146,771 (46.67) | -- | -- | 0.51 | (0.49, 0.53) |
| MC/ADRD/90 patients§ | 220,046 (100.00) | ||||
| MM | 134,116 (60.95) | 4.92 | (4.63, 5.22) | 2.42 | (2.34, 2.51) |
| Non-MM | 85,930 (39.05) | 2.22 | (2.08, 2.37) | 1.13 | (1.08, 1.18) |
* The full list of 118 QCSs with their frequency and ORs (95% CI) are provided in Appendix G (Table G.2)
† Typical reference group consisting of a random 20% sample of 2018-2019 admissions. Patients with Metastatic Cancers, ADRD, age ≥90 years, or on dialysis were not included in the typical reference group.; Non-multimorbid reference group drawn from the same random 20% sample comprised of patients who do not have any QCSs as well as patients without Metastatic Cancers, ADRD, or age ≥90 years. Dashes (--) indicate a population odds ratio of 1, reflecting the fact that the comparison groups are a random split of one population
‡Ranked by lower 95% CI of odds for 30-day mortality relative to the medical condition-specific typical reference population
§Frequency and odds of 30-day mortality for multimorbid and non-multimorbid patients with Metastatic Cancers, ADRD, or age ≥90 years are further detailed in Appendix G (Table G.4)
Table 3.
Frequency and Odds of 30-day Mortality with Qualifying Comorbidity Sets for Pneumonia Patients
| Qualifying Comorbidity Sets* |
Frequency (%) 385,219 (100.00) |
Ref. non-MM† (N=41,667) |
Ref. typical† (N=92,169) |
||
|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||
| A. Top 10 QCS listed by highest to lowest frequency | |||||
| Acute Heart or Respiratory Failure | Acute Renal Failure | 72,024 (18.70) | 7.02 | (6.66, 7.40) | 2.24 | (2.18, 2.30) |
| Protein-Calorie Malnutrition | 65,651 (17.04) | 7.40 | (7.02, 7.80) | 2.33 | (2.26, 2.39) |
| Thrombocytopenia and Other Hematological Disorders | Acute Heart or Respiratory Failure | 45,920 (11.92) | 7.44 | (7.04, 7.85) | 2.38 | (2.31, 2.45) |
| Acute Myocardial Infarction | 44,534 (11.56) | 6.32 | (5.99, 6.68) | 2.03 | (1.97, 2.09) |
| Acute Heart or Respiratory Failure | CKD Stage 4–5 or Dialysis | 32,157 (8.35) | 7.02 | (6.64, 7.43) | 2.25 | (2.18, 2.33) |
| Thrombocytopenia and Other Hematological Disorders | Acute Renal Failure | 27,175 (7.05) | 7.60 | (7.18, 8.05) | 2.45 | (2.36, 2.53) |
| Sepsis or Septic Shock | Acute Heart or Respiratory Failure | Cardiac Arrhythmias | 25,336 (6.58) | 7.09 | (6.69, 7.52) | 2.26 | (2.18, 2.34) |
| Pressure Ulcer of Skin | 24,347 (6.32) | 8.09 | (7.63, 8.57) | 2.57 | (2.48, 2.66) |
| Acute Heart or Respiratory Failure | Chronic Non-Pressure Skin Ulcers | 23,400 (6.07) | 7.29 | (6.87, 7.73) | 2.33 | (2.25, 2.42) |
| Liver Disease | 18,672 (4.85) | 7.68 | (7.21, 8.19) | 2.45 | (2.35, 2.55) |
| B. Top 5 QCS listed by highest to lowest odds of mortality‡ | |||||
| Cardiac Arrhythmias | CKD Stage 4-5 or Dialysis | Other Trauma | 3072 (0.80) | 8.46 | (7.69, 9.31) | 2.76 | (2.55, 3.00) |
| Cardiac Arrhythmias | Chronic Non-Pressure Skin Ulcers | Complications of Implants or Grafts | 4071 (1.06) | 8.54 | (7.82, 9.32) | 2.75 | (2.56, 2.96) |
| Sepsis or Septic Shock | Cardiac Arrhythmias | CKD Stage 4-5 or Dialysis | 8654 (2.25) | 8.05 | (7.50, 8.64) | 2.60 | (2.46, 2.74) |
| Sepsis or Septic Shock | Cardiac Arrhythmias | Chronic Non-Pressure Skin Ulcers | 7327 (1.90) | 8.07 | (7.50, 8.69) | 2.60 | (2.46, 2.76) |
| Pressure Ulcer of Skin | 24,347 (6.32) | 8.09 | (7.63, 8.57) | 2.57 | (2.48, 2.66) |
| C. Patients for MM definition development and MC/ADRD/90 patients | |||||
| Patients for MM definition development | 385,219 (100.00) | ||||
| MM | 220,093 (57.14) | 5.17 | (4.91, 5.44) | 1.65 | (1.62, 1.69) |
| Non-MM | 165,126 (42.87) | -- | -- | 0.32 | (0.31, 0.33) |
| MC/ADRD/90 patients§ | 434,165 (100.00) | ||||
| MM | 295,663 (68.10) | 9.99 | (9.50, 10.51) | 3.11 | (3.05, 3.18) |
| Non-MM | 138,502 (31.90) | 2.85 | (2.70, 3.01) | 0.93 | (0.90, 0.96) |
* The full list of 51 QCSs with their frequency and ORs (95% CI) are provided in Appendix G (Table G.3)
† Typical reference group consisting of a random 20% sample of 2018-2019 admissions. Patients with Metastatic Cancers, ADRD, age ≥90 years, or on dialysis were not included in the typical reference group.; Non-multimorbid reference group drawn from the same random 20% sample comprised of patients who do not have any QCSs as well as patients without Metastatic Cancers, ADRD, or age ≥90 years. Dashes (--) indicate a population odds ratio of 1, reflecting the fact that the comparison groups are a random split of one population
‡Ranked by lower 95% CI of odds for 30-day mortality relative to the medical condition-specific typical reference population
§Frequency and odds of 30-day mortality for multimorbid and non-multimorbid patients with Metastatic Cancers, ADRD, or age ≥90 years are further detailed in Appendix G (Table G.4)
AMI Patients
Among 186,012 AMI patients, Acute Heart or Respiratory Failure was the most common QCS with N= 42,342 (22.76%) (ORnon 10.62 [95% CI= 9.78, 11.53], ORtypical 3.32 [3.19, 3.47]). The QCS with the highest odds of mortality was “Liver Disease plus Acute Renal Failure” (henceforth written as Liver Disease | Acute Renal Failure) (N= 1295 [0.70%]), ORnon 15.45 [13.22, 18.06]), ORtypical 4.98 [4.35, 5.70] (Table 1).
HF Patients
Among 314,510 HF patients, Protein-Calorie Malnutrition was the most common QCS with N= 32,385 (10.30%) (ORnon 4.83 [4.53, 5.15], ORtypical 2.39 [2.29, 2.49]). The highest odds of mortality were among patients with Thrombocytopenia and Other Hematological Disorders | Chronic Non-Pressure Skin Ulcers | Home Oxygen Use (N= 2394 [0.76%]), ORnon 4.93 [4.34, 5.59], ORtypical 2.44 [2.17, 2.73] (Table 2).
Pneumonia Patients
The most common QCS among 385,219 pneumonia patients was Acute Heart or Respiratory Failure | Acute Renal Failure with N= 72,024 (18.70%) (ORnon 7.02 [6.66, 7.40], ORtypical 2.24 [2.18, 2.30]). The highest odds of mortality were among patients with Cardiac Arrhythmias | Chronic Kidney Disease (CKD) Stage 4–5 or Dialysis | Other Trauma (N= 3072 [0.80%]), ORnon 8.46 [7.69, 9.31] and ORtypical 2.76 [2.55, 3.00] (Table 3).
Metastatic Cancers/ADRD/Age ≥90 Patients
As expected, MC/ADRD/90 patients had far higher odds of mortality relative to both the non-multimorbid (ORnon= 7.89 [7.26, 8.58]) and typical (ORtypical= 2.52 [2.41, 2.63]) reference groups. For the patients who were multimorbid, the ORnon for mortality was higher than for non-multimorbid patients. Among AMI patients (10.68 versus 2.81) (Table 1), HF patients (4.92 versus 2.22) (Table 2), and pneumonia patients (9.99 versus 2.85) (Table 3). The ORnon and ORtypical for each of these three conditions are shown in Appendix G, Table G.1-3.
Comparing New Versus Conventional Definition of Multimorbidity
We found that the conventional definition (≥2 comorbidities) labeled almost all patients as multimorbid, while 30-day mortality rates were not very high. In contrast, our new multimorbidity definition (≥1 QCS) labeled only about 50% of patients as multimorbid for each medical condition, with considerably higher mortality rates (Table 4). Comparing ≥1 QCS versus ≥2 comorbidities multimorbidity definitions, we found that, for AMI patients, 46.74% versus 86.61% (p value <0.0001) were labeled as multimorbid while mortality rates were 14.99% versus 9.48%, respectively (p value<0.0001). Similarly, for HF patients, 53.33% versus 97.68% (p value <0.0001) were labeled as multimorbid with mortality rates 9.88% versus 7.03% (p value<0.0001). For pneumonia patients 57.13% versus 90.87% (p value<0.0001) were labeled as multimorbid and mortality rates were 18.37% versus 13.19% (p value<0.0001).
Table 4.
Comparing rates of Multimorbidity and 30-Day Mortality by ≥1 QCS-Based Definitions Versus ≥2 Comorbidities Definition
| AMI | HF | Pneumonia | ||||
|---|---|---|---|---|---|---|
| Categorizations | Frequency (%) | Mortality rate | Frequency (%) | Mortality rate | Frequency (%) | Mortality rate |
| All patients | 186,012 (100.00) | 8.45% | 314,510 (100.00) | 6.90% | 385,219 (100.00) | 12.14% |
| New Multimorbidity definition: 0 QCS versus ≥1 QCS | ||||||
| Non-MM (QCS=0) | 99,063 (53.26) | 2.70% | 146,771 (46.67) | 3.50% | 165,126 (42.87) | 3.83% |
| MM (QCS≥1) | 86,949 (46.74) | 14.99% | 167,739 (53.33) | 9.88% | 220,093 (57.13) | 18.37% |
| Standard Multimorbidity Definition: 0-1 Comorbidities versus ≥2 Comorbidities | ||||||
| 0–1 Comorbidities | 24,911 (13.39) | 1.79% | 7300 (2.32) | 1.81% | 35,157 (9.13) | 1.71% |
| ≥2 Comorbidities | 161,101 (86.61) | 9.48% | 307,210 (97.68) | 7.03% | 350,062 (90.87) | 13.19% |
| Effect of number of QCSs on Mortality | ||||||
| 0 QCS | 99,063 (53.26) | 2.70% | 146,771 (46.67) | 3.50% | 165,126 (42.87) | 3.83% |
| 1 QCS | 33,117 (17.80) | 10.60% | 39,310 (12.50) | 7.26% | 90,997 (23.62) | 11.33% |
| 2 QCSs | 17,902 (9.62) | 14.74% | 19,300 (6.14) | 8.14% | 41,507 (10.78) | 18.87% |
| 3 QCSs | 10,741 (5.77) | 17.73% | 14,489 (4.61) | 8.61% | 26,096 (6.77) | 21.17% |
| 4 QCSs | 6,833 (3.67) | 18.94% | 13,411 (4.26) | 8.73% | 16,401 (4.26) | 24.51% |
| ≥5 QCSs | 18,356 (9.87) | 20.09% | 81,229 (25.83) | 11.99% | 45,092 (11.71) | 28.29% |
| Effect of number of Comorbidities on Mortality* | ||||||
| 0 Comorbidities | 3,888 (2.09) | 1.65% | 1,385 (0.44) | 1.66% | 10,633 (2.76) | 1.02% |
| 1 Comorbidity | 21,023 (11.30) | 1.81% | 5,915 (1.88) | 1.84% | 24,524 (6.37) | 2.01% |
| 2 Comorbidities | 28,984 (15.58) | 2.89% | 13,749 (4.37) | 2.33% | 36,708 (9.53) | 3.54% |
| 3 Comorbidities | 29,493 (15.86) | 5.08% | 23,553 (7.49) | 2.77% | 45,117 (11.71) | 5.93% |
| 4 Comorbidities | 26,392 (14.19) | 7.24% | 33,008 (10.50) | 3.79% | 48,880 (12.69) | 8.53% |
| ≥5 Comorbidities | 76,232 (40.98) | 14.46% | 236,900 (75.32) | 8.17% | 219,357 (56.94) | 17.33% |
*Based on the number of comorbidities included in the development of our new multimorbidity definition (23 for AMI, 26 for HF, 24 for Pneumonia)
Thirty-day mortality rates among patients with one or more QCSs were still higher than those among patients with five or more comorbidities. For AMI (14.99% versus 14.46%), HF (9.88% versus 8.17%), and pneumonia (18.38% versus 17.33%), suggesting that the types of comorbidities were more important than their quantity.
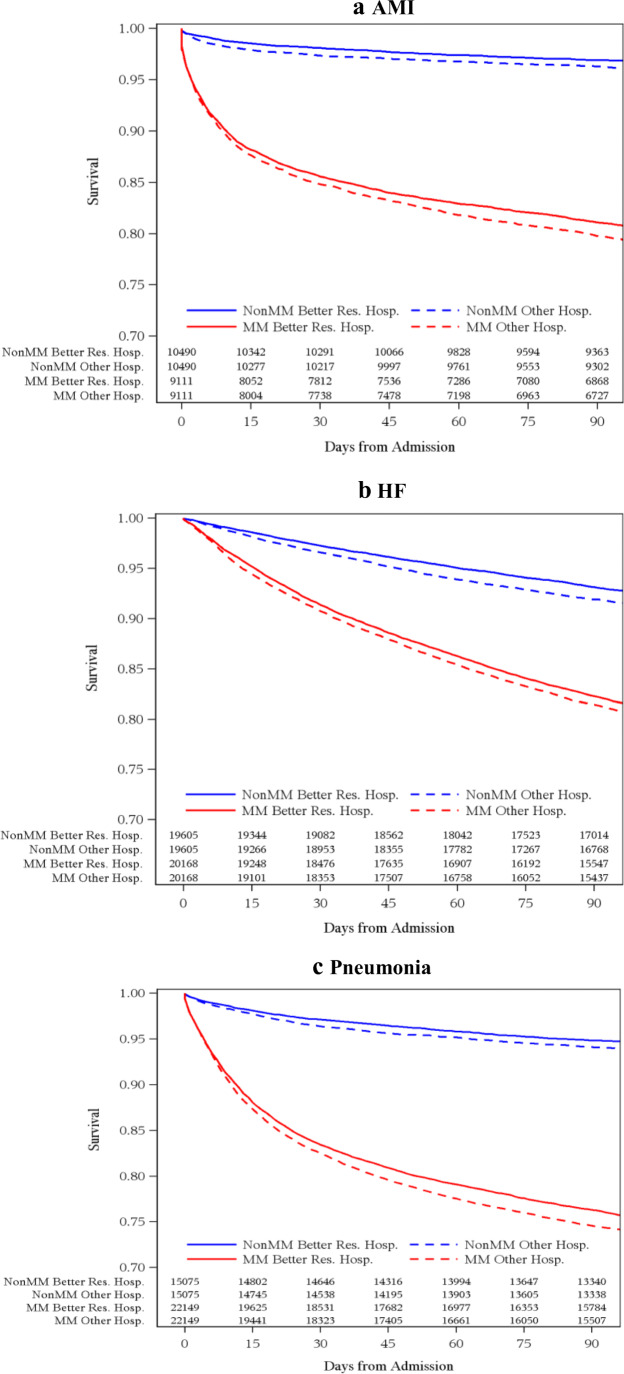
External Validation of Multimorbidity in Better-Resourced Hospitals
To externally validate our multimorbidity definitions, we matched patients in better-resourced hospitals to similar controls in other hospitals (Appendix H). The 30-day mortality rates were significantly lower among patients in better-resourced hospitals compared to those in other hospitals. Among AMI patients, the difference in mortality was −0.76% (95% CI −1.27%, −0.265) (Table 5 and Fig. 1a). Similarly, for HF and pneumonia patients, the differences were −0.64% (−0.96%, −0.31%) and −0.87% (−1.29%, −0.44%), respectively (Table 5 and Fig. 1b and 1c). Among the multimorbid population, HF (−0.61% [−1.17, −0.06]) and pneumonia (−0.96% [−1.63, −0.29]) patients had better 30-day survival in better-resourced hospitals. There were no significant differences for multimorbid AMI patients.
Table 5.
Comparing Differences in 30-Day Mortality Rates for Multimorbid (MM) and Non-Multimorbid patients in Better-Resourced and Other Hospitals*
| Conditions | N | Better-resourced hospitals (%) | Other hospitals (%) | Difference in rates (%) | 95% CI |
|---|---|---|---|---|---|
| 30-day mortality (Primary outcome) | |||||
| AMI | |||||
| OVERALL | 19,601 | 7.73 | 8.49 | −0.76† | (−1.27, −0.26) |
| MM | 9111 | 14.42 | 15.19 | −0.77 | (−1.75, 0.21) |
| Non-MM | 10,490 | 1.92 | 2.67 | −0.75‡ | (−1.16, −0.34) |
| MM vs. Non-MM | -- | -- | -- | −0.02 | (−1.07, 1.04) |
| HF | |||||
| OVERALL | 39,773 | 5.72 | 6.36 | −0.64‡ | (−0.96, −0.31) |
| MM | 20,168 | 8.62 | 9.24 | −0.61§ | (−1.17, −0.06) |
| Non-MM | 19,605 | 2.74 | 3.41 | −0.66‡ | (−1.01, −0.32) |
| MM vs. Non-MM | -- | -- | -- | 0.05 | (−0.59, 0.69) |
| Pneumonia | |||||
| OVERALL | 37,224 | 11.03 | 11.89 | −0.87‡ | (−1.29, −0.44) |
| MM | 22,149 | 16.57 | 17.52 | −0.96† | (−1.63, −0.29) |
| Non-MM | 15,075 | 2.89 | 3.62 | −0.73‡ | (−1.13, −0.33) |
| MM vs. Non-MM | -- | -- | -- | −0.23 | (−1.00, 0.55) |
| 90-day mortality | |||||
| AMI | |||||
| OVERALL | 19,601 | 10.45 | 11.40 | −0.95‡ | (−1.52, −0.39) |
| MM | 9111 | 18.90 | 20.24 | −1.34§ | (−2.44, −0.24) |
| Non-MM | 10,490 | 3.10 | 3.72 | −0.62§ | (−1.10, −0.14) |
| MM vs. Non-MM | -- | -- | -- | −0.72 | (−1.94, 0.49) |
| HF | |||||
| OVERALL | 39,773 | 12.39 | 13.41 | −1.03‡ | (−1.47, −0.58) |
| MM | 20,168 | 17.74 | 18.56 | −0.82§ | (−1.53, −0.11) |
| Non-MM | 19,605 | 6.87 | 8.10 | −1.23‡ | (−1.73, −0.73) |
| MM vs. Non-MM | -- | -- | -- | 0.41 | (−0.46, 1.27) |
| Pneumonia | |||||
| OVERALL | 37,224 | 16.18 | 17.53 | −1.35‡ | (−1.86, −0.84) |
| MM | 22,149 | 23.68 | 25.44 | −1.77‡ | (−2.57, −0.97) |
| Non-MM | 15,075 | 5.17 | 5.91 | −0.74† | (−1.23, −0.25) |
| MM vs. Non-MM | -- | -- | -- | −1.03§ | (−1.98, −0.08) |
* MM vs. Non-MM rows showing Difference-in-Difference of multimorbid versus non-multimorbid mortality rates
§ p value<0.05 †p value<0.01 ‡p value<0.001
Figure 1.
Kaplan-Meier survival plots by multimorbidity (MM) status and hospital type for AMI (a), HF (b), and pneumonia (c).
When examining 90-day mortality we found similar results. (Table 5 and Fig. 1a, b and c). The difference-in-difference for multimorbid versus non-multimorbid patients in better-resourced versus other hospitals was significantly lower for pneumonia patients −1.03 (−1.98, −0.08, p value=0.03) (Table 5 and Fig. 1c). It was not significant for AMI and HF patients.
DISCUSSION
Various definitions have been proposed for multimorbidity in medical patients.10–14,58–60 However, our new data-driven QCS-based definitions for hospitalized patients have the following strengths. First, unlike definitions based on simply counting comorbidities, a QCS combines specific comorbidities that substantially increase the risk for patients with a specific medical condition. A QCS that doubles the risk of death for pneumonia patients may not do so for AMI and HF, Second, our QCS definitions identify a smaller, more intelligible, and an actionable subset of patients with a substantially elevated risk of death, which could be incorporated into research and clinical algorithms. These new QCS-based definitions identified approximately half of AMI, HF, and pneumonia patients as multimorbid, compared to about 90% of patients who had two or more comorbidities. Third, our new QCS-based multimorbidity definitions now incorporate indicators for functional status. We found that the indicators Home Oxygen Use and Home Hospital Bed or Wheelchair Use were important components for QCSs, consistent with studies showing an association between chronic diseases and functional status.61–63 Furthermore, the methods described in this paper and its supplementary material could be used to expand multimorbidity definitions for hospitalized patients with different medical conditions or surgical procedures.
In the development of multimorbid definitions, we excluded a considerable number of high-risk patients with metastatic cancers, ADRD, or age ≥90 years. However, for the validation, we applied our multimorbidity definitions to all patients including these high-risk patients. Our new definitions were also able to identify the elevated risk even among these high-risk patients.
Additionally, we found that 30-day mortality rates among multimorbid patients with ≥1 QCS were significantly higher than those for patients with ≥2 comorbidities. In fact, we showed that for AMI, HF, and pneumonia, mortality rates among patients with one or more QCSs were higher than those among patients with five or more comorbidities.
Our new multimorbidity definitions were further validated by analyzing outcomes among multimorbid versus non-multimorbid patients in an external dataset. In this dataset, we defined better-resourced hospitals using teaching hospital status, nursing skill mix, and condition-specific patient volume. Recent studies have shown better outcomes among patients in teaching hospitals64–66 and hospitals with better nursing resources.67 Similarly, we found that better-resourced hospitals, compared to other hospitals, had significantly better mortality outcomes for multimorbid versus non-multimorbid pneumonia patients, although this did not reach significance for AMI or HF patients.
The study has some limitations. Our sample was restricted to fee-for-service Medicare claims, because it was not possible to determine the prevalence of comorbidities in the Medicare managed care population. Our data included not only inpatient Medicare records but other Medicare claims from outpatient, carrier/Part B, Skilled Nursing Facility, and Home Health Agency files. As medical care advances over time, the risks posed by various QCSs to AMI, HF, and pneumonia patients may change, requiring occasional updates to the multimorbidity definitions.
In summary, for AMI, HF, and pneumonia patients, our data-driven approach defined multimorbidity in terms of a relatively short but high-risk list of QCSs comprised of one, two, or three comorbidities or functional status indicators, that were associated with a large increase in the risk of 30-day mortality. Current practice labels almost all older patients admitted for AMI, HF, and pneumonia patients as multimorbid, thereby failing to identify patients at especially high-risk or to make useful and actionable distinctions among patients. In contrast, our QCS-based definitions identify a more specific proportion of patients who face a substantial excess risk of death. Thus, our new multimorbidity definitions may help providers and policymakers make better-informed decisions for these complex, vulnerable patients and more effectively use such information to provide tailored interventions.
Supplementary Information
(DOCX 265 kb)
Funding
This research was funded by a grant from the National Institute on Aging (Grant # R01AG060928).
Declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tinetti ME, Green AR, Ouellet J, Rich MW, Boyd C. Caring for patients with multiple chronic conditions. Ann Intern Med. 2019;170:199–200. doi: 10.7326/M18-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity Guiding principles for the care of older adults with multimorbidity: An approach for clinicians. J Am Geriatr Soc. 2012;60:E1–E25. doi: 10.1111/j.1532-5415.2012.04188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: Implications for pay for performance. JAMA. 2005;294:716–24. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Medicare & Medicaid Services. Chronic Conditions among Medicare Beneficiaries, Chartbook (2012) Edition. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/Downloads/2012Chartbook.pdf. .
- 5.Marengoni A, Rizzuto D, Wang HX, Winblad B, Fratiglioni L. Patterns of chronic multimorbidity in the elderly population. J Am Geriatr Soc. 2009;57:225–30. doi: 10.1111/j.1532-5415.2008.02109.x. [DOI] [PubMed] [Google Scholar]
- 6.Starfield B, Kinder K. Multimorbidity and its measurement. Health Policy. 2011;103:3–8. doi: 10.1016/j.healthpol.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Tooth L, Hockey R, Byles J, Dobson A. Weighted multimorbidity indexes predicted mortality, health service use, and health-related quality of life in older women. J Clin Epidemiol. 2008;61:151–9. doi: 10.1016/j.jclinepi.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Silber JH, Reiter JG, Rosenbaum PR, et al. Defining multimorbidity in older surgical patients. Med Care. 2018;56:701–10. doi: 10.1097/MLR.0000000000000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suls J, Bayliss EA, Berry J, et al. Measuring multimorbidity: selecting the right instrument for the purpose and the data source. Med Care. 2021;59:743–56. doi: 10.1097/MLR.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorace J, Wong HH, Worrall C, Kelman J, Saneinejad S, MaCurdy T. The complexity of disease combinations in the Medicare population. Popul Health Manag. 2011;14:161–6. doi: 10.1089/pop.2010.0044. [DOI] [PubMed] [Google Scholar]
- 11.Noyes K, Liu H, Temkin-Greener H. Medicare capitation model, functional status, and multiple comorbidities: model accuracy. Am J Manag Care. 2008;14:679–90. [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman B, Jiang HJ, Elixhauser A, Segal A. Hospital inpatient costs for adults with multiple chronic conditions. Med Care Res Rev. 2006;63:327–46. doi: 10.1177/1077558706287042. [DOI] [PubMed] [Google Scholar]
- 13.Ajmera M, Wilkins TL, Findley PA, Sambamoorthi U. Multimorbidity, mental illness, and quality of care: preventable hospitalizations among Medicare beneficiaries. Int J Family Med. 2012;2012:1–10. doi: 10.1155/2012/823294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider KM, O'Donnell BE, Dean D. Prevalence of multiple chronic conditions in the United States' Medicare population. Health Qual Life Outcomes. 2009;7:82. doi: 10.1186/1477-7525-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starfield B, Lemke KW, Herbert R, Pavlovich WD, Anderson G. Comorbidity and the use of primary care and specialist care in the elderly. Ann Fam Med. 2005;3:215–22. doi: 10.1370/afm.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salive ME. Multimorbidity in older adults. Epidemiol Rev. 2013;35:75–83. doi: 10.1093/epirev/mxs009. [DOI] [PubMed] [Google Scholar]
- 17.Uijen AA, van de Lisdonk EH. Multimorbidity in primary care: prevalence and trend over the last 20 years. Eur J Gen Pract. 2008;14(Suppl 1):28–32. doi: 10.1080/13814780802436093. [DOI] [PubMed] [Google Scholar]
- 18.Radner H, Yoshida K, Smolen JS, Solomon DH. Multimorbidity and rheumatic conditions-enhancing the concept of comorbidity. Nat Rev Rheumatol. 2014;10:252–6. doi: 10.1038/nrrheum.2013.212. [DOI] [PubMed] [Google Scholar]
- 19.Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases--a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci. 2011;66:301–11. doi: 10.1093/gerona/glq208. [DOI] [PubMed] [Google Scholar]
- 20.Freisling H, Viallon V, Lennon H, et al. Lifestyle factors and risk of multimorbidity of cancer and cardiometabolic diseases: a multinational cohort study. BMC Med. 2020;18:5. doi: 10.1186/s12916-019-1474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landi F, Liperoti R, Russo A, et al. Disability, more than multimorbidity, was predictive of mortality among older persons aged 80 years and older. J Clin Epidemiol. 2010;63:752–9. doi: 10.1016/j.jclinepi.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Schram MT, Frijters D, van de Lisdonk EH, et al. Setting and registry characteristics affect the prevalence and nature of multimorbidity in the elderly. J Clin Epidemiol. 2008;61:1104–12. doi: 10.1016/j.jclinepi.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Min LC, Wenger NS, Fung C, et al. Multimorbidity is associated with better quality of care among vulnerable elders. Med Care. 2007;45:480–8. doi: 10.1097/MLR.0b013e318030fff9. [DOI] [PubMed] [Google Scholar]
- 24.Glynn LG, Valderas JM, Healy P, et al. The prevalence of multimorbidity in primary care and its effect on health care utilization and cost. Fam Pract. 2011;28:516–23. doi: 10.1093/fampra/cmr013. [DOI] [PubMed] [Google Scholar]
- 25.Kenzik KM, Kent EE, Martin MY, Bhatia S, Pisu M. Chronic condition clusters and functional impairment in older cancer survivors: a population-based study. J Cancer Surviv. 2016;10:1096–103. doi: 10.1007/s11764-016-0553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei MY, Kabeto MU, Langa KM, Mukamal KJ. Multimorbidity and physical and cognitive function: performance of a new multimorbidity-weighted index. J Gerontol A Biol Sci Med Sci. 2018;73:225–32. doi: 10.1093/gerona/glx114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei MY, Luster JE, Ratz D, Mukamal KJ, Langa KM. Development, validation, and performance of a new physical functioning-weighted multimorbidity index for use in administrative data. J Gen Intern Med. 2021;36:2427–33. doi: 10.1007/s11606-020-06486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huntley AL, Johnson R, Purdy S, Valderas JM, Salisbury C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med. 2012;10:134–41. doi: 10.1370/afm.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alemi F, Levy CR, Kheirbek RE. The multimorbidity index: a tool for assessing the prognosis of patients from their history of illness. EGEMS (Wash DC). 2016;4:1235. doi: 10.13063/2327-9214.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aubert CE, Schnipper JL, Roumet M, et al. Best definitions of multimorbidity to identify patients with high health care resource utilization. Mayo Clin Proc Innov Qual Outcomes. 2020;4:40–49. doi: 10.1016/j.mayocpiqo.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vela E, Cleries M, Monterde D, et al. Performance of quantitative measures of multimorbidity: a population-based retrospective analysis. BMC Public Health. 2021;21:1881. doi: 10.1186/s12889-021-11922-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Healthcare Cost and Utilization Project (HCUP) (2018) Most Frequent Principal Diagnoses for Inpatient Stays in U.S. Hospitals, Rockville, MD: Agency for Healthcare Research and Quality, 2021. (Statistical Brief #277). Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb277-Top-Reasons-Hospital-Stays-2018.pdf. Accessed September 13, 2022.
- 33.Research Data Assistance Center (2021) CMS Virtual Research Data Center (VRDC). Available at: https://www.resdac.org/cms-virtual-research-data-center-vrdc. .
- 34.Pope GC, Kautter J, Ellis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25:119–41. [PMC free article] [PubMed] [Google Scholar]
- 35.Chronic Conditions Warehouse (2020) Chronic Condition Warehouse User Guide. Medicare Risk Score Files.Version 1.1. Available at: https://www2.ccwdata.org/documents/10280/19002246/ccw-medicare-risk-score-user-guide.pdf. Accessed February 24, 2022.
- 36.American Medical Association . ICD-10-CM 2017: The complete official code book. Chicago, IL: American Medical Association; 2016. [Google Scholar]
- 37.Medicode (Firm) ICD-9-CM: International classification of diseases, 9th revision, clinical modification. Salt Lake City, Utah: Medicode; 1996. [Google Scholar]
- 38.Health Alliance. RxHCC Model (2020) Available at: https://provider.healthalliance.org/coding-counts-post/rxhcc-model/. .
- 39.Calderon-Larranaga A, Vetrano DL, Ferrucci L, et al. Multimorbidity and functional impairment-bidirectional interplay, synergistic effects and common pathways. J Intern Med. 2019;285:255–71. doi: 10.1111/joim.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan A, Wallace E, O'Hara P, Smith SM. Multimorbidity and functional decline in community-dwelling adults: a systematic review. Health Qual Life Outcomes. 2015;13:168. doi: 10.1186/s12955-015-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cesari M, Perez-Zepeda MU, Marzetti E. Frailty and multimorbidity: different ways of thinking about geriatrics. J Am Med Dir Assoc. 2017;18:361–64. doi: 10.1016/j.jamda.2016.12.086. [DOI] [PubMed] [Google Scholar]
- 42.Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health. 2018;3:e323–e32. doi: 10.1016/S2468-2667(18)30091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wetmore JB, Yan H, Hu Y, Gilbertson DT, Liu J. Factors associated with withdrawal from maintenance dialysis: a case-control analysis. Am J Kidney Dis. 2018;71:831–41. doi: 10.1053/j.ajkd.2017.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mariano C, Lund JL, Peacock Hinton S, Htoo P, Muss H, Reeder-Hayes KE. Evaluating the association between adjuvant chemotherapy and function-related adverse events among older patients with early stage breast cancer. J Geriatr Oncol. 2017;8:242–48. doi: 10.1016/j.jgo.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 45.VanDerwerker CJ, Gregory CM, Simpson KN. Using inferred mobility status to estimate the time to major depressive disorder diagnosis post-spinal cord injury. Arch Phys Med Rehabil. 2020;101:658–66. doi: 10.1016/j.apmr.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jencks SF, Daley J, Draper D, Thomas N, Lenhart G, Walker J. Interpreting hospital mortality data. The role of clinical risk adjustment. JAMA. 1988;260:3611–16. doi: 10.1001/jama.1988.03410240081036. [DOI] [PubMed] [Google Scholar]
- 47.Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with an acute myocardial infarction. Circulation. 2006;113:1683–92. doi: 10.1161/CIRCULATIONAHA.105.611186. [DOI] [PubMed] [Google Scholar]
- 48.Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113:1693–701. doi: 10.1161/CIRCULATIONAHA.105.611194. [DOI] [PubMed] [Google Scholar]
- 49.CMS.gov (2007) Frequently Asked Questions (FAQs). Implementation and Maintenance of CMS Mortality Measures for AMI & HF, . Available at: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/HospitalMortalityAboutAMI_HF.pdf. Accessed September 13, 2022.
- 50.Zhao Q, Small DS, Rosenbaum PR. Cross-screening in observational studies that test many hypotheses. J Am Stat Assoc. 2018;113:1070–84. doi: 10.1080/01621459.2017.1407770. [DOI] [Google Scholar]
- 51.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Nat Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 52.Rosenbaum PR. Design of Observational Studies. 2nd ed. New York, NY: Springer; 2020. Part II: Matching; pp. 191–312. [Google Scholar]
- 53.Rosenbaum PR. Modern algorithms for matching in observational studies. Annu Rev Stat Appl. 2020;7:143–76. doi: 10.1146/annurev-statistics-031219-041058. [DOI] [Google Scholar]
- 54.Silber JH, Rosenbaum PR, McHugh MD, et al. Comparison of the value of nursing work environments in hospitals across different levels of patient risk. JAMA Surg. 2016;151:527–36. doi: 10.1001/jamasurg.2015.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silber JH, Rosenbaum PR, Niknam BA, et al. Comparing outcomes and costs of surgical patients treated at major teaching and nonteaching hospitals: a national matched analysis. Ann Surg. 2020;271:412–21. doi: 10.1097/SLA.0000000000003602. [DOI] [PubMed] [Google Scholar]
- 56.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. 3. New York: John Wiley & Sons; 2003. Chapter 13. The Analysis of Data from Matched Samples. Section 13.1. Matched Pairs: Dichotomous Outcome; pp. 374–496. [Google Scholar]
- 57.Institute SAS. Version 9.4 of the Statistical Analytic Software System for UNIX. Cary, NC: SAS Institute, Inc.; 2013. [Google Scholar]
- 58.Librero J, Peiro S, Ordinana R. Chronic comorbidity and outcomes of hospital care: Length of stay, mortality, and readmission at 30 and 365 days. J Clin Epidemiol. 1999;52:171–9. doi: 10.1016/S0895-4356(98)00160-7. [DOI] [PubMed] [Google Scholar]
- 59.Gross CP, Guo Z, McAvay GJ, Allore HG, Young M, Tinetti ME. Multimorbidity and survival in older persons with colorectal cancer. J Am Geriatr Soc. 2006;54:1898–904. doi: 10.1111/j.1532-5415.2006.00973.x. [DOI] [PubMed] [Google Scholar]
- 60.Lochner KA, Cox CS. Prevalence of multiple chronic conditions among Medicare beneficiaries, United States, 2010. Prev Chronic Dis. 2013;10:E61. doi: 10.5888/pcd10.120137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jindai K, Nielson CM, Vorderstrasse BA, Quinones AR. Multimorbidity and functional limitations among adults 65 or older, NHANES 2005-2012. Prev Chronic Dis. 2016;13:E151. doi: 10.5888/pcd13.160174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quinones AR, Markwardt S, Botoseneanu A. Multimorbidity combinations and disability in older adults. J Gerontol A Biol Sci Med Sci. 2016;71:823–30. doi: 10.1093/gerona/glw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stenholm S, Westerlund H, Head J, et al. Comorbidity and functional trajectories from midlife to old age: the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2015;70:332–8. doi: 10.1093/gerona/glu113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silber JH, Rosenbaum PR, Niknam B, et al. Comparing outcomes and costs of medical patients treated at major teaching and nonteaching hospitals: a national matched analysis. J Gen Intern Med. 2020;35:743–52. doi: 10.1007/s11606-019-05449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ayanian JZ, Weissman JS. Teaching hospitals and quality of care: A review of the literature. Milbank Q. 2002;80:569–93. doi: 10.1111/1468-0009.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burke LG, Frakt AB, Khullar D, Orav EJ, Jha AK. Association between teaching status and mortality in US hospitals. JAMA. 2017;317:2105–13. doi: 10.1001/jama.2017.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lasater KB, McHugh MD, Rosenbaum PR, et al. Evaluating the costs and outcomes of hospital nursing resources: a matched cohort study of patients with common medical conditions. J Gen Intern Med. 2021;36:84–91. doi: 10.1007/s11606-020-06151-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 265 kb)